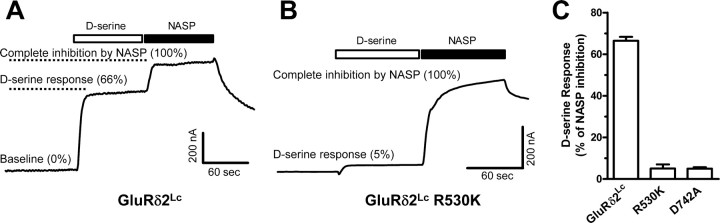
Figure 2.
A, Representative two-electrode voltage-clamp recording of d-serine-mediated current responses at GluRδ2Lc expressed in Xenopus oocytes. Applications of saturating concentrations of d-serine (30 mm) and the channel blocker 1-naphthyl acetyl spermine (NASP; 100 μm) are indicated by the bars above the current trace. At the beginning of the recording, the baseline currents are predominantly mediated by the spontaneously active GluRδ2Lc. The spontaneously active GluRδ2Lc currents are inhibited upon application of a maximally effective concentration of d-serine. Application of the channel blocker NASP results in complete inhibition (100%) of the spontaneously active GluRδ2Lc current. B, Representative two-electrode voltage-clamp recording of current responses at GluRδ2Lc R530K. d-Serine at 30 mm minimally reduced spontaneously active currents at GluRδ2Lc R530K (5%). C, d-Serine at 30 mm reduced the spontaneously active current by 66 ± 2% at GluRδ2Lc (N = 7) relative to complete inhibition (100%) by NASP. The d-serine response (i.e., d-serine-induced inhibition) was decreased to 5 ± 2% for R530K (N = 9) and 5 ± 1% for D742A (N = 9) relative to complete inhibition (100%) by NASP. In the structure of the GluRδ2 agonist-binding domain in complex with d-serine, the guanidinium group of R530 interacts with the α-carboxyl group of d-serine, and the carboxylate group of D742 interacts with the α-amino group of d-serine.