Figure 7.
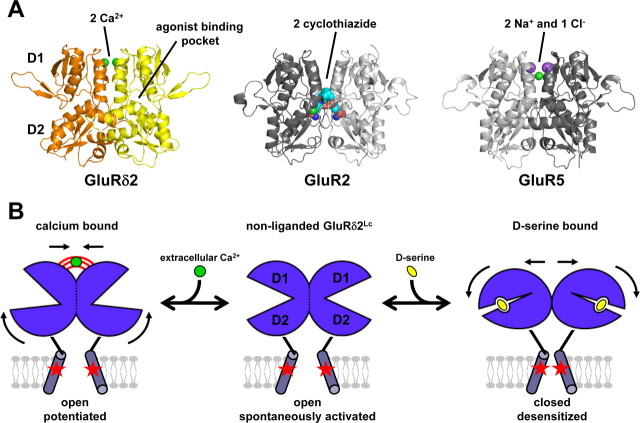
A, The results from the present study suggest that the dimer interface controls the function of GluRδ2, and stability of this interface is influenced by Ca2+ (green spheres). This feature is shared across the iGluR family. Desensitization of AMPA receptors is inhibited by binding of positive modulators at the dimer interface, such as cyclothiazide binding to GluR2 (shown as spacefill, PDB code 1LBC). Desensitization of kainate receptors is regulated by binding of anions and cations at the dimer interface, such as two sodium (purple spheres) and one chloride (green) binding to GluR5 (PDB code 3C32). Mutations that stabilize the dimer interface attenuate desensitization of both AMPA and kainate receptors. B, Hypothetical model that illustrates the opposing effects of d-serine and Ca2+ on GluRδ2Lc (lurcher mutation indicated by red stars). d-Serine binding in the agonist-binding pocket between D1 and D2 and subsequent domain closure can result in a rearrangement of the dimer interface formed by two agonist-binding domains. d-Serine binding to spontaneously active GluRδ2Lc results in a rearrangement of the dimer interface. This rearrangement at the dimer interface results in desensitization by repositioning the transmembrane helices to a nonconducting conformation, where the ion channel is closed. However, binding of Ca2+ attenuates d-serine-induced desensitization by stabilizing the dimer interface in a conformation that permits opening of the ion channel. The Ca2+-stabilized conformation of the dimer interface permits increased opening of the ion channel and thus results in potentiation of the spontaneously active current.