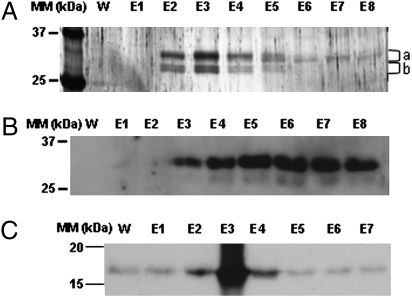
Fig. 1.
Identification of PII-interacting proteins in leaf chloroplast extracts. Soluble proteins from purified chloroplasts were loaded onto a PII-affinity resin. Unbound proteins were removed by washing with 50 mL of buffer A. Lane W shows the absence of protein at the end of the wash process. Bound proteins were eluted with 5 mM 2-OG (8 × 0.5 mL fractions corresponding to lanes E1–E8). In control experiments omitting Mg-ATP or with NAGK fixed to the affinity resin, these proteins were not bound. (A) Eluted proteins were TCA-precipitated and subjected to SDS/PAGE (12% acrylamide) and silver staining. (B) Bound proteins were eluted with 0.5 mM 2-OG and, after SDS/PAGE, submitted to Western blotting with BCCP antibodies. (C) In a reverse approach, soluble biotin-containing proteins from intact chloroplasts were fixed to immobilized avidin and their interaction with recombinant PII was tested in the presence of Mg-ATP. PII was eluted with 5 mM 2-OG and fractions were evaluated for PII content by Western blotting using PII antibodies. For explanation of a and b, see Table 1 and Fig. S1.