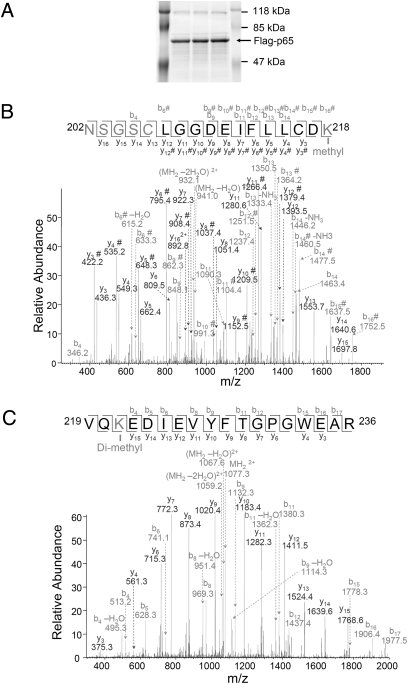
Fig. 2.
Mass spectrometry (MS) shows that p65 is methylated on K218 and K221 on NF-κB activation. (A) GelCode blue-stained gel, showing that p65 is immunoprecipitated as a single strong band. The same samples were loaded into multiple lanes. (B) Analysis of tryptic peptides derived from p65 suggests that K218 is monomethylated. The pure single band was digested in the gel and samples were analyzed by LC-MS/MS. A mass shift of +14 was observed spanning peptide 202–218. Tandem MS analysis further suggested that K218 on the C-terminal side of the peptide is modified. Another +14 mass shift was also observed on the N-terminal side, suggesting an additional methylation within the N-terminal four residues. (C) Analysis of tryptic peptides derived from p65 suggests that K221 of p65 is dimethylated. A mass shift of +28 was observed spanning peptide 219–236. Tandem MS analysis further suggested that K221 on the N-terminal is dimethylated.