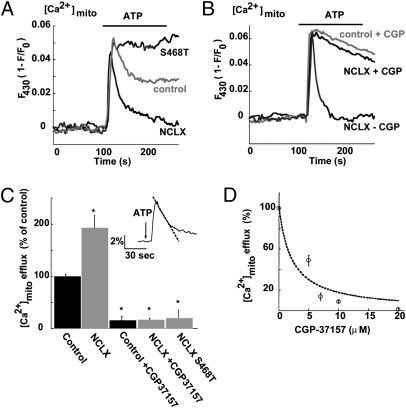
Fig. 3.
Expression of NCLX enhances mitochondrial Ca2+ efflux that is blocked by mutation in the catalytic site of NCLX and by the mitochondrial exchanger inhibitor CGP-37157. (A) SHSY-5Y cells transfected with plasmids encoding the mouse NCLX, NCLX-S468T mutant, or the vector (control) were cotransfected to express RP-mt. Cells were superfused with ATP-containing Ringer solution (40 μM at the indicated time) while monitoring mitochondrial Ca2+ fluorescence. Note that enhancement of NCLX levels increased mitochondrial Ca2+ efflux, and the mutant was not only inactive but also exerted a dominant-negative effect on endogenous activity. (B) Illustration of the same experimental paradigm as in A repeated in control or NCLX-expressing cells in the presence of the mitochondrial exchanger inhibitor CGP-37157 (10 μM). Similar inhibition of Ca2+ efflux is seen in both. (C) Averaged mitochondrial Ca2+ efflux rates (n = 9; **P < 0.01). (Inset) Experimental paradigm for Ca2+ efflux rate measurement based on determination of the initial rate of the mitochondrial Ca2+ efflux phase. (D) Dose-dependence analysis of the effect of CGP-37157 on mitochondrial Ca2+ efflux in cells expressing NCLX. Mitochondrial Ca2+ efflux rate was measured in the presence of the indicated concentrations of CGP-37157 and presented as percentage of the rate measured in its absence. The dashed line is a fit of the data to the equation I = Io / [1+([CGP] / IC50)].