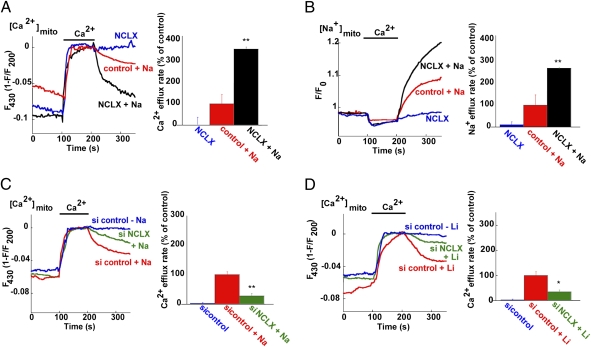
Fig. 5.
The mitochondrial Na+- or Li+-dependent Ca2+ exchange is mediated by NCLX. (A) Traces and rates of Na+-dependent mitochondrial Ca2+ efflux. Mitochondrial Ca2+ levels were recorded by monitoring RP-mt fluorescence in HEK-293 cells overexpressing either the human NCLX isoform (NCLX) or vector alone (control) and coexpressing RP-mt. Experiments were conducted on digitonin-permeabilized cells (see Materials and methods). Mitochondrial Ca2+ uptake was induced by superfusion with Na+-free solution (replaced by NMDG+) containing Ca2+ (60 μM). Ca2+ efflux was monitored following superfusion in Ca2+-free solution in the presence or absence of Na+. Note that the Ca2+ efflux was strictly Na+-dependent and was enhanced by the expression of NCLX (n = 8, **P < 0.01). (B) Traces and rates of Na+-dependent mitochondrial Na+ influx. Mitochondrial Na+ levels were measured in cells loaded with the Na+-sensitive dye CoroNa Red (see Materials and methods) as in A. Note the enhanced and reciprocal nature of the Na+ and Ca2+ transport rates, mediated by mitochondrial NCLX (n = 8, **P < 0.01). (C) Traces and rates of Na+-dependent mitochondrial Ca2+ efflux in cells transfected with either siNCLX or a scrambled siRNA (si control). The same experimental paradigm described in A was applied. Silencing of NCLX eliminated mitochondrial Na+-dependent Ca2+ efflux (n = 11, **P < 0.01). (D) Traces and rates of Li+-dependent mitochondrial Ca2+ efflux in cells transfected with either siNCLX or si control, using Li+-containing solution (replacing Na+; n = 11; *P < 0.05). Silencing of NCLX also diminished Li+-dependent Ca2+ efflux.