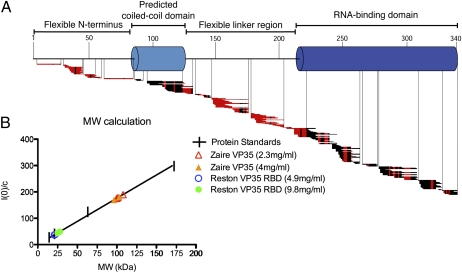
Fig. 4.
Biophysical characterization of VP35. (A) Ten-second amide hydrogen–deuterium exchange map for select VP35 peptides of a near full-length Zaire VP35. Red horizontal bars indicate fast-exchanging amides; black bars indicate stretches of no exchange. (B) Molecular weight calculation of Zaire VP35 (triangles) and the Reston VP35 RBD (circles). Our Zaire VP35 expression construct encodes a 37-k Da molecule. SAXS analysis indicates a ∼100 kDa mass, consistent with the oligomeric (probably trimeric) nature of VP35 in solution. The RBD construct (here, indicated by Reston) encodes a molecule ∼20 kDa in size. SAXS indicates a ∼20–25 kDa mass, consistent with the monomeric nature of the RBD in the absence of dsRNA.