Figure 1.
Collagen Peptide Binding by the DDR2 DS Domain
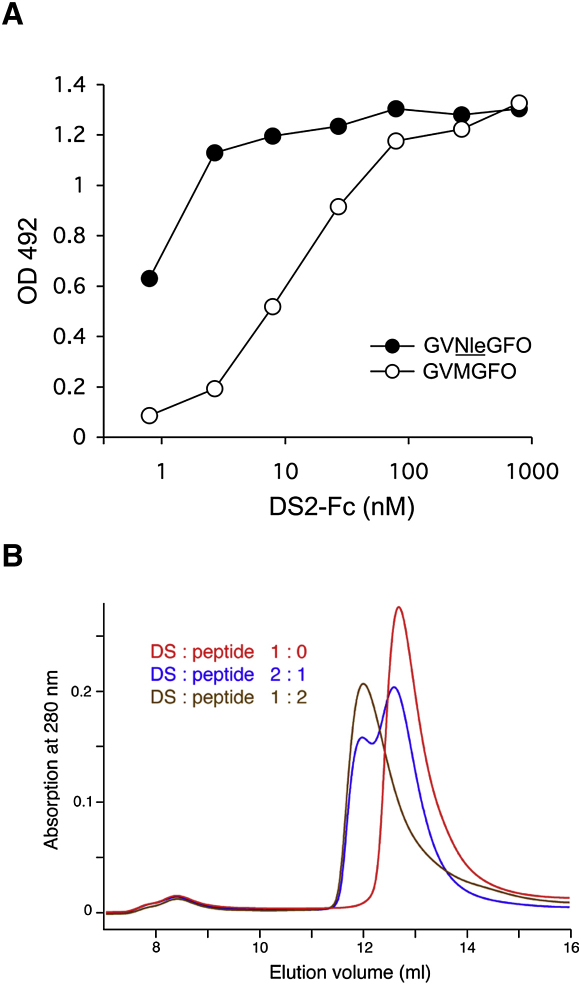
(A) Solid-phase binding assay with recombinant DS2-Fc protein (Leitinger, 2003) added to 96-well plates coated with triple-helical collagen peptides at 10 μg/ml: GPC-(GPP)5-GPRGQOGVXGFO-(GPP)5-GPC-NH2, where X is either methionine or norleucine. Shown is a representative of three independent experiments, each performed in duplicate.
(B) Analytical size exclusion chromatograms of the free DDR2 DS domain and its complex with the triple-helical collagen peptide Ac-GPOGPOGPOGPR-GQOGVNleGFOGPOGPOG-NH2. The DS domain and peptide were mixed in the indicated molar ratios. A globular molecular mass standard of 29 kDa, carbonic anhydrase, elutes at 12.3 ml from this column.