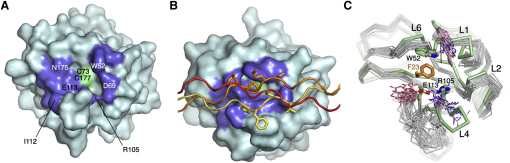
Figure 4.
Structural Changes in the DDR2 DS Domain upon Collagen Binding
(A) Surface representation of the free DDR2 DS domain in solution (Ichikawa et al., 2007). The collagen-binding residues identified by this study are in green (disulfide) and blue (all other residues). Selected residues are labeled.
(B) Surface representation of the DS domain in complex with the collagen peptide (yellow, leading chain; orange, middle chain; red, trailing chain). The side chains of the GVMGFO motif are shown as sticks. The view direction and coloring of the DS domain surface are the same as in (A).
(C) Superposition of the NMR ensemble of the free DDR2 DS domain (Ichikawa et al., 2007) (gray Cα traces; Trp52, Arg105, and Glu113 side chains in pink) and the crystal structure of the DDR2 DS-collagen (green, DS domain; orange, Phe23 of the collagen middle chain). The structures were superimposed using 43 Cα atoms of the DS domain β-barrel (rmsd 0.82 Å). Model 5 of the NMR ensemble is most similar overall to the crystal structure (rmsd 1.8 Å) and was taken as the reference. Selected residues and loops are labeled.