Abstract
Trichloroethylene (TCE) is the most frequently reported organic groundwater contaminant in the United States. It is controversial whether gestational TCE exposure causes congenital heart defects. The basis for TCE’s proposed cardiac teratogenicity is not well understood. We previously showed that chick embryos exposed to 8 ppb TCE during cardiac morphogenesis have reduced cardiac output and increased mortality. To further investigate TCE’s cardioteratogenic potential, we exposed in ovo chick embryos to TCE and evaluated the heart thereafter. Significant mortality was observed following TCE exposures of 8–400 ppb during a narrow developmental period (Hamburger-Hamilton [HH] stages 15–20, embryo day ED2.3–3.5) that is characterized by myocardial expansion, secondary heart looping, and endocardial cushion formation. Of the embryos that died, most did so between ED5.5 and ED6.5. Echocardiography of embryos at ED5.5 found that TCE-exposed hearts displayed significant functional and morphological heterogeneity affecting heart rate, left ventricular mass, and wall thickness. Individual embryos were identified with cardiac hypertrophy as well as with hypoplasia. Chick embryos exposed to 8 ppb TCE at HH17 that survived to hatch exhibited a high incidence (38%, p < 0.01, n = 16) of muscular ventricular septal defects (VSDs) as detected by echocardiography and confirmed by gross dissection; no VSDs were found in controls (n = 14). The TCE-induced VSDs may be secondary to functional impairments that alter cardiac hemodynamics and subsequent ventricular foramen closure, an interpretation consistent with recent demonstrations that TCE impairs calcium handling in cardiomyocytes. These data demonstrate that TCE is a cardiac teratogen for chick.
Keywords: trichloroethylene, biphasic dose response, cardiac teratogen, chick embryo, ventricular septal defects, echocardiography
Trichloroethylene (TCE, C2HCl3) is a common environmental contaminant in groundwater, soils, and airborne emissions. It is a widely used industrial degreaser and solvent and is an intermediate in chemical manufacturing. Human exposure to TCE and its metabolites occurs through exposure to contaminated water or ambient air in both home settings and the workplace. The U.S. Environmental Protection Agency (EPA) currently allows a maximum contaminant level (MCL) of 5 μg/l (5 ppb) in drinking water, but it has been found to contaminate groundwater in the United States at concentrations as high as 27,300 ppb (Board on Environmental Studies and Toxicology [BEST], 2006). The World Health Organization (2008) guideline for TCE in drinking water is 20 ppb.
A controversial question is whether TCE and its metabolites are cardiac teratogens. Epidemiological studies of human exposure are conflicting and have experimental limitations. Some find no association (Agency for Toxic Substances and Disease Registry, 1998; Bove et al., 1995; Lagakos et al., 1986), whereas others find a significant correlation between gestational TCE exposure and congenital heart defects in the offspring (Bove et al., 2002; Goldberg et al., 1990; Yauck et al., 2004). The most commonly reported defects were valvuloseptal and included muscular and membranous ventricular septal defects (VSDs), atrial septal defects (ASDs), and pulmonary and aortic stenosis.
Animal studies of TCE and its metabolites are equally conflicting. Some rat studies find no cardiac dysmorphologies following gestational exposure to high-dose oral (Fisher et al., 2001) or inhalational TCE (Carney et al., 2006; Dorfmueller et al., 1979; Healy et al., 1982; Schwetz et al., 1975). Others find defects consistent with those observed in the epidemiological studies, with the preponderance again affecting valvuloseptal development (Dawson et al., 1993; Johnson et al., 2003).
In contrast, TCE is clearly a cardiac teratogen in avians. TCE exposures (1–100 ppm/egg) during organogenesis (ED1–6) caused high embryo mortality, edema, and blood pooling, suggestive of impaired heart function (Bross et al., 1983; Elovaara et al., 1979). Exposures in the low parts per billion range also increased mortality and caused congenital heart defects involving not only valvuloseptal formation, including VSDs, ASDs, truncus arteriosus, and atrioventricular canal anomalies, but also muscular and chambering defects (Loeber et al., 1988). In both whole-embryo explant culture (Mishima et al., 2006) and a well-defined in vitro assay of cushion morphogenesis (Boyer et al., 2000), TCE exposures in the parts per million range reduced the ability of cardiac endothelial cells to transform into the mesenchymal cells that largely constitute the valves and septa. Lower TCE exposures (8 ppb) during the valvuloseptal morphogenesis period caused significant embryo mortality (Drake et al., 2006b), hyperplastic cushions, and reduced cardiac output, whereas exposure during the earlier events of heart specification were largely without adverse effect (Drake et al., 2006a).
Taken together, these findings suggested that one or more events during the period of valvuloseptal morphogenesis have the greatest vulnerability to TCE with respect to cardiac outcome and embryo survival. To better understand how TCE adversely affects cardiogenesis, here, we show that a fairly narrow period of heart development is the most sensitive to disruption by TCE. During this period, embryo survival is most adversely affected at TCE concentrations that are slightly above the EPA’s MCL of 5 ppb (BEST, 2006). Using a novel approach of real-time high-resolution cardiac imaging, we found that a subset of TCE-treated embryos had abnormal cardiac structure and function. TCE-exposed chicks that survived to hatch had a high incidence of VSDs as identified by Doppler imaging. Our findings suggest that the prior emphasis upon static measures could have missed functional cardiac changes and subtle dysmorphologies and thus may have underestimated TCE’s cardiac teratogenicity.
MATERIALS AND METHODS
Animals.
Fertile White Leghorn chicken eggs (Hyline strain W36, Spencer, IA) were incubated until the desired stage of development according to the criteria of Hamburger and Hamilton (HH, 1951). Most embryos did not develop past ED6.5 and did not experience pain or suffering. Hatched chicks were treated humanely and were anesthetized during all experimental procedures. Protocols were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee.
Embryonic TCE exposure.
Embryo treatment with TCE or PBS vehicle was identical to that of Drake et al. (2006b) except that a single injection was administered into the yolk center at one of the following developmental stages: HH13, HH15, HH17, HH20, or HH24 (ED2.0, ED2.3, ED2.5, ED3.3, or ED4.25, respectively). TCE doses were 0.2, 4, 40, 200, or 2000 nmol per egg with 200 μl injection volume and resulted in approximate TCE concentrations of 0.4, 8, 80, 400, and 4000 ppb per egg, respectively. Injected eggs were sealed and reincubated.
Echocardiography.
Echocardiography was performed on TCE- and PBS-treated embryos at ED5.5 using a Visual Sonics Vevo 770 ultrasonograph with a 55-mHg transducer and an ex ovo culture setup (McQuinn et al., 2007). Cross-sectional B-mode images were obtained with all four chambers clearly visible. M-mode images were also obtained across ventricles from this view at the largest diameter of both chambers. Doppler studies were performed across the mitral and tricuspid openings and the interventricular foramen and were used to measure cardiac outflows. A separate set of 1-day-old hatched chicks were imaged using an Acuson Sequoia ultrasonograph (Siemens) with a 15L8 transducer as detailed in Harris et al. (2002). Chicks were sedated by facemask administration of 1% isoflurane and maintained on a heated pad. The chest was shaved and prewarmed coupling gel applied. Transmitral velocities were measured using Doppler pulse-wave imaging from a four-chamber view.
End diastolic and systolic left ventricular (LV) diameters and anterior wall (AW) and posterior wall (PW) thickness were measured online from M-mode images using the leading edge-to-leading edge convention. All parameters were measured over at least three consecutive cardiac cycles. LV fractional shortening was calculated as [(LV diameterdiastole − LV diametersystole)/LV diameterdiastole] × 100. Calculated LV mass was estimated using the formula [1.05 × ((PWdiastole + AWdiastole + LV diameterdiastole)3 − (LV diameterdiastole)3)]. For embryos, LV volumes were measured from a cross-sectional view to obtain the largest volume of all four chambers. The interior edges were traced by hand at end diastole and end systole for at least three separate images. The inner length of the major axis was measured in both systole and diastole. Endocardial volume was calculated using: 4π/3 × endocardial major/2 × (endocardial area/π(endocardial major/2))2. For hatched chicks, right ventricular (RV) diameter was measured from an apical four-chamber view at the widest diameter. Relative wall thickness was calculated as 2 × PWdiastole/LV diameterdiastole. At both ages, heart rate was determined from at least three consecutive intervals from the pulse-wave Doppler tracings of the LV outflow tract. Imaging in Doppler mode identified VSDs by documenting blood flow across the interventricular septum.
Following echocardiography of hatched chicks, 20% KCl in PBS was injected into the LV lumen to arrest the beating heart at end diastole. The LV and RV were dissected and weighed. The anterior LV wall was opened, and the presence or absence of a VSD was confirmed by direct evaluation.
Immunohistochemistry.
Paraffin sections were prepared from HH28 embryos fixed in 4% paraformaldehyde. Proliferating myocytes were labeled in HH24 embryos with BrdU immunohistochemistry as previously described (Drake et al., 2006b). We determined the percentage of proliferating myocytes by counting numbers of BrdU-labeled cells relative to total propidium iodide–labeled cells within the ventricles.
Statistical analyses.
Survival data were subjected to logistic regression analysis. Echocardiography data and physical parameters were analyzed using Student’s t-test assuming equal variance as appropriate. The VSD incidence was analyzed by chi-square analysis. A p value < 0.05 was the critical level of significance for all experiments.
RESULTS
TCE Exposure Causes Embryo Mortality and Cardiac Dysfunction
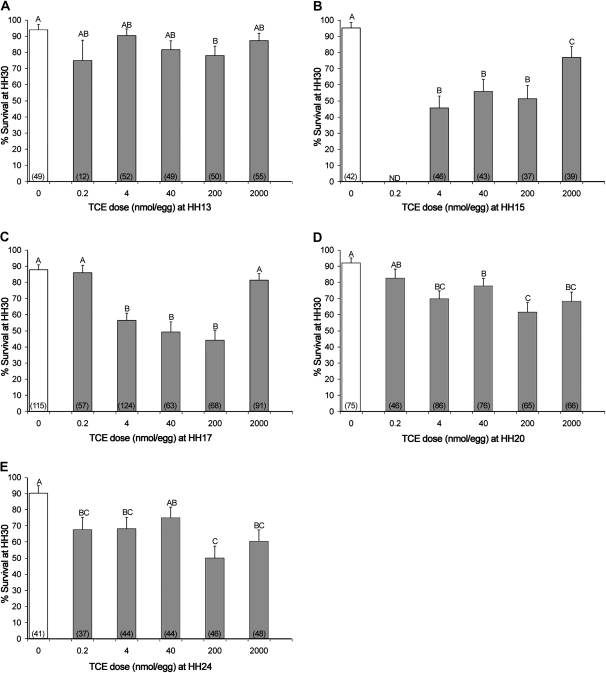
To define the event(s) of avian cardiogenesis sensitive to disruption by TCE, in ovo embryos received a single TCE dose during defined events of cardiomorphogenesis previously identified as a window of TCE sensitivity (e.g., developmental stages HH13–24). During this period, the heart initiates valvuloseptal morphogenesis and significantly increases cardiomyocyte proliferation, trabeculation, and output as it transitions from a tubular to a chambered structure (Person et al., 2005; Sedmera and McQuinn, 2008). Exposure at HH13 had, at best, a modest impact on embryo survival to HH30, wherein 200 nmol per egg caused a small but significant reduction in survival compared with the vehicle control (Fig. 1A). In contrast, TCE exposure at HH15 or HH17 significantly reduced embryo survival by HH30. This effect was greatest for doses between 4 and 200 nmol per egg (Figs. 1B and 1C) and was lesser or ineffectual at lower (0.2 nmol/egg) or higher doses (2000 nmol/egg), suggestive of a nonmonotonic dose response at these stages. TCE administration at HH20 and HH24 resulted in less mortality, which occurred across the range of doses ≥4 nmol per egg. Survival in control embryos was similar at all treatment stages averaging 88–95%.
FIG. 1.
Embryo survival following TCE exposure at distinct developmental times. Mean ± SE of percent embryo survival to HH30 (ED6.5) following TCE exposure at (A) HH13, ED2.0; (B) HH15, ED2.3; (C) HH17, ED2.5; (D) HH20, ED3.5; and (E) HH24, ED4.5. Results are pooled from at least four independent experiments. The total number of embryos evaluated is indicated in parenthesis. Values with identical letters do not significantly differ (p > 0.05) from one another as ascertained by logistic regression analysis.
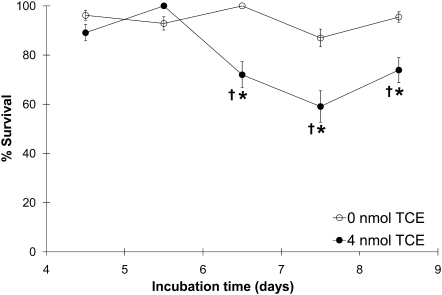
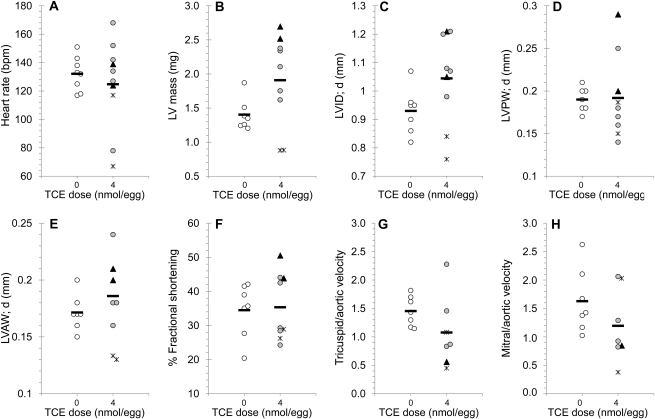
A time course (Fig. 2) established that embryos exposed to the most potent treatment regimen, 4 nmol TCE per egg at HH17, began to die at ED6.5 (HH28–30), which was 3–4 days after TCE administration. Although the overall appearance of TCE-exposed hearts at HH28, shortly before mortality onset, was unremarkable with respect to ventricular and atrial morphology, trabeculation, and cushion appearance (Figs. 3A–D), echocardiography of the TCE-exposed hearts revealed substantial heterogeneity in cardiac structure and function. For example, TCE-treated embryos had important variations in heart rate, LV mass, and ventricular wall thickness compared with controls (Figs. 4A–H). Two hearts (triangles, Fig. 4) had characteristics consistent with hypertrophy, including enlarged ventricular mass and wall thickness. Two additional hearts (asterisks, Fig. 4) were substantially reduced in size and featured thin ventricular walls and profoundly reduced heart rates suggesting hypoplasia. Electrocardiographic videos of these hearts are presented in Supplementary figure 1. These changes were preceded at ED4.5 by similarly divergent frequencies of myocyte proliferation in the ventricles (Fig. 3E). Because the ED5.5 embryos otherwise matched the morphological criteria for HH28, the differences were not attributable to developmental delay. Doppler imaging of TCE-treated embryos revealed a trend toward reduced blood inflow velocities at the tricuspid (RV) and mitral (LV) valves but not at the aortic outflow valve (AoV; Figs. 4G and 4H). As the echocardiography was a terminal procedure, we could not determine which phenotypes were inconsistent with continued survival.
FIG. 2.
Time course of embryonic death after TCE exposure at HH17. Percentage of embryos that survived to ED4.5–8.5 (HH24, HH28, HH30, HH32, and HH35) after exposure to 0 or 4 nmol per egg TCE at HH17. Values represent the mean ± SE of two to four independent experiments and comprising 14–52 embryos per data point. *Comparison of 0 versus 4 nmol by logistic regression analysis; p = 0.045 (ED6.5), p = 0.040 (ED7.5), and p = 0.076 (ED8.5). †Comparison of survival at various days following 4 nmol treatment versus ED4.5 by logistic regression analysis; p = 0.063 (ED6.5), p = 0.005 (ED7.5), and p = 0.099 (ED8.5). No time effect on survival was found for 0 nmol treatment.
FIG. 3.
Morphological and proliferative assessment of TCE-treated embryos. Representative sections of HH28 embryos stained with hematoxylin-eosin and treated with 0 nmol (A) or 4 nmol (B) TCE at HH17. (A and B) Four-chamber view at 40× magnification and (C and D) 100× view of a right ventricle and its trabeculation. (E) Percentage of proliferating myocytes in ED4.5 (HH24) ventricles.
FIG. 4.
Echocardiography of TCE-exposed embryos at HH28. Distribution of individual heart rate (A), calculated LV mass (B), LV internal diameter during diastole (C), LV PW and AW thickness during diastole (D and E), fractional shortening (F), tricuspid to aortic velocity ratio (G), and mitral to aortic velocity ratio (H) in HH28 embryos treated with 0 or 4 nmol per egg TCE at HH17. The difference from the group mean was greater in TCE-treated embryos than in control embryos, as determined by Student’s t-test. Values were p = 0.090 (A), 0.012 (B), 0.049 (D), 0.029 (E), and 0.082 (G); all other values were p > 0.1. ▴, hyperplastic hearts; *, hypoplastic hearts; and  , mean.
, mean.
TCE Exposure Causes VSDs
We also analyzed the long-term consequences of TCE exposure in hatched chicks that had been exposed to 4 nmol TCE or vehicle at HH17. Survival beyond ED7.5 was equivalent in both groups. The gross appearance of TCE-exposed hearts in hatched chicks was indistinguishable from vehicle-treated hearts. Total heart weight, LV and RV weight, and external diameter of the great vessels were normal (Table 1). Functional parameters (Table 2) were also normal except for a significant increase in heart rate (p < 0.002) and a commensurate increase in blood flow through the tricuspid valve (p < 0.002). There was no change in the fractional shortening of each ventricle, suggesting that cardiac output was normal. Calculated LV mass, as well as thickness of the LV and RV walls, was also normal.
TABLE 1.
Physical Parameters at 3-Day Posthatch
| Parameter | 0 nmol TCE | 4 nmol TCE |
| BW (g) | 39.4 ± 2.6 (8) | 39.7 ± 5.2 (10) |
| LV mass (mg) | 185 ± 19 (3) | 190 ± 20 (9) |
| RV mass (mg) | 44.3 ± 7.9 (4) | 49.3 ± 8.0 (8) |
| LV mass/BW (mg/g) | 4.83 ± 0.48 (3) | 4.77 ± 0.41 (9) |
| RV mass/BW (mg/g) | 1.14 ± 0.18 (4) | 1.23 ± 0.18 (8) |
| Pulmonary artery (external diameter) | 238 ± 32 (4) | 244 ± 22 (13) |
| Aorta (external diameter) | 208 ± 64 (5) | 234 ± 41 (16) |
Note. Mean ± SD, N is indicated in parenthesis. No values were significantly different (p > 0.05) as assessed by Student’s t-test. BW, body weight.
TABLE 2.
Functional Parameters at 1-Day Posthatch
| Parameter | 0 nmol TCE | 4 nmol TCE |
| BW (g) | 40.7 ± 3.1 | 39.7 ± 5.2 |
| Heart rate (beats/min) | 277 ± 37 | 349 ± 52* |
| Tricuspid velocity (max) | 55.4 ± 7.0 | 66.4 ± 6.5* |
| Pulmonary valve velocity (max) | 55.2 ± 13.6 | 56.0 ± 17.6 |
| PWd (mm) | 1.00 ± 0.22 | 0.97 ± 0.07 |
| PWs (mm) | 1.59 ± 0.34 | 1.47 ± 0.18 |
| AWd (mm) | 0.92 ± 0.19 | 0.95 ± 0.05 |
| AWs (mm) | 1.67 ± 0.32 | 1.62 ± 0.18 |
| LVDd (mm) | 3.37 ± 0.27 | 3.24 ± 0.54 |
| LVDs (mm) | 1.69 ± 0.29 | 1.64 ± 0.41 |
| LVFS% | 50 ± 6 | 50 ± 5 |
| RVDd (mm) | 2.47 ± 0.37 | 2.45 ± 0.56 |
| RVDs (mm) | 1.07 ± 0.27 | 1.10 ± 0.32 |
| RVFS% | 57.7 ± 7 | 55 ± 20 |
| LV mass (mg) | 120 ± 44 | 110 ± 28 |
| LV mass/BW (mg/g) | 2.92 ± 1.06 | 2.84 ± 0.99 |
Note. Mean ± SD of functional parameters assessed by echocardiography, N = 10 per group. AWs and AWd, anterior wall thickness in systole and diastole; BW, body weight; LVDs and LVDd, LV diameter in systole and diastole; LVFS% and RVFS%, % fractional shortening of LV and RV; LV mass, calculated LV mass; PWs and PWd, posterior wall thickness in systole and diastole; RVDs and RVDd, RV diameter in systole and diastole.
*p = 0.002 by Student’s t-test.
However, 37.5% (6/16) of hatched chicks exposed to 4 nmol TCE at HH17 displayed VSDs. Doppler mode imaging clearly captured the abnormal movement of blood from the LV into the RV through a hole across the muscular portion of interventricular septum, a flow pattern consistent with a VSD (Figs. 5A and 5B). No VSDs were identified in vehicle-treated chicks (0/14, p < 0.01). The presence or absence of VSDs was confirmed by direct dissection (Figs. 5C and 5D). All the TCE-associated VSDs were located in the upper third of the muscular region of the interventricular septum; no membranous VSDs were found.
FIG. 5.
VSDs in TCE-exposed hatched chicks. (A and B) Doppler images of VSD (arrow) in two different chicks exposed to 4 nmol TCE at HH17. (C and D) Anatomical view of a VSD in a TCE-treated heart seen from the RV (C) and LV (D) aspects.
DISCUSSION
This study independently confirms that TCE is a cardiac teratogen. TCE administered to avian embryos at doses near the EPAs MCL caused high embryonic mortality, functional dysmorphologies, and, in animals that survived to hatch, a significant frequency of VSDs. In humans, while not immediately life threatening, such muscular VSDs when left untreated ultimately reduce cardiac efficiency, stimulate hypertrophy, and eventually become incompatible with life. The chick embryo was most sensitive to TCE-induced mortality during a relatively narrow exposure window at a time when the heart tube initiates structural and functional changes that enable it to adapt to an increasing workload caused by the embryo’s rapid growth. This delineation of TCE’s critical window is consistent with proposals that a teratogen’s effects should be selective and specific. Its identification also will facilitate elucidation of TCE’s teratogenic mechanism in the chick and will assist evaluation of its potential teratogenicity in other species.
Mechanism of TCE-Induced VSDs
In chick, the interventricular foramen completely closes by ED8 (HH34); chicks hatch at ED21. Thus, the muscular VSDs seen here cannot be attributed to a transient closure delay that will spontaneously heal later in life. The interventricular septum consists of a muscular portion derived from the myocardial wall and a smaller membranous portion that arises from endocardial cushion fusion with the apical muscular septum. The VSDs detected here all involved the upper region of the muscular septum. Muscular VSDs have been reported in studies of TCE and its metabolites in humans, rodents, and chicks (Dawson et al., 1993; Johnson et al., 1998; Loeber et al., 1988; Smith et al., 1989). One cause of such defects is a failure of muscular septation itself. Another major contributor is cardiac hemodynamics, which alter both intraventricular pressures and shear stress and the subsequent shaping and closure of the ventricular and outflow tract foramens (Hogers et al., 1999; Hove et al., 2003; Sedmera et al., 1999). Thus, the VSDs seen here, and perhaps the outflow tract defects reported by others, could have resulted from direct effects of TCE on cushion formation or they could be secondary to effects on myocyte contractility and/or muscular uplifting during formation of the septum primum.
With respect to cushion morphogenesis, TCE does not affect the early events during endothelial cell activation (Boyer et al., 2000), a finding consistent with the lack of effect seen here for HH13 exposure as well as our previous finding of little effect of TCE when administered during cardiac specification (Drake et al., 2006a). However, subsequent cushion transformation events are sensitive to alteration by TCE. High TCE doses impair endocardiocyte migration into the cushion matrix and their mesenchymal differentiation (Boyer et al., 2000; Mishima et al., 2006). Our finding that TCE’s effects are greatest when administered during HH15 or HH17, when endocardiocyte migration and transformation begin, is consistent with such a target. The TCE doses used here cause hyperplasia of cushion mesenchymal cells (Drake et al., 2006a,b). Cushion hyperplasia could alter the precise alignment between the ventricular chambers and cushions necessary for proper septal closure.
TCE exposure also disrupts events of myocardial morphogenesis. During the TCE exposure period studied here, the myocardium transitions from a tubular to a chambered heart. Cardiomyocyte proliferation and differentiation, as well as trabeculation and ventricular wall compaction, all serve to increase cardiac output and meet the accelerating vascular demands of a rapidly growing embryo (Sedmera and McQuinn, 2008). Extracardiac populations including the endothelium, epicardium, and neural crest, also contribute to this expansion. Halogenated aliphatic hydrocarbons including TCE can act as myocardial depressants and arrhythmic agents, dampening calcium transients and thus reducing contractile force (Caldwell et al., 2008; Hoffmann et al., 1994). In cultured myocytes, TCE reduces the expression of Serca2a and the ryanodine receptor (Caldwell et al., 2008; Collier et al., 2003), which govern calcium release and reuptake by the sarcoplasmic reticulum during the cardiomyocyte contraction cycle. This is accompanied by significant impairments in calcium homeostasis, with reductions in both peak calcium release and cytosolic calcium clearance at TCE levels only slightly greater (10 ppb) than those studied here. Such impairments reduce contractility and contribute to cardiac failure (Ikeda et al., 2008; Porter et al., 2003). Therefore, disrupted calcium handling may account for the cardiac dysmorphologies seen at ED5.5. Hypertrophy is the heart’s attempt to increase output in response to functional challenge by increasing cellular myofibril content and thus ventricular wall thickness, chamber volume, and ejection fraction; in the embryo, increased myocyte proliferation also contributes to the hypertrophy response (deAlmeida et al., 2007). Hearts that poorly compensate have thin ventricular walls and reduced heart rates and output and ultimately fail. A similar mix of hypertrophic and dilated hearts occurs in a genetic model of cardiac failure (Cote et al., 2003). The abnormal phenotypes seen in TCE-treated embryos at ED5.5 likely represent attempts to improve cardiac output in response to impaired contractility. A possible impairment of extracardiac populations may also be contributory, and this merits investigation.
Cardiac contractility and the attendant increases in blood flow and intraventricular pressure also contribute to the closure of the intraventricular foramen, and experimental manipulation of cardiac flow produces VSDs (Hogers et al., 1999; Hove et al., 2003; Sedmera et al., 1999). We previously reported reduced flow velocities at the level of the AV valves and dorsal aorta in HH24 (ED4.5) embryos exposed to TCE at HH15 or HH17 (Drake et al., 2006b), a finding consistent with the reduced AV velocities documented here at ED5.5. Because the TCE-exposed AV and outflow tract cushions had similar levels of hypercellularity (Drake et al., 2006b), the presence of normal aortic valve velocity (AoV), despite lower AV velocities, could represent compensatory activity of the contractile conotruncus (Keller et al., 1991). Alternately, the reduced AV inflow may reflect impaired ventricular relaxation due to abnormal calcium handling (Keller et al., 1991) or could be due to lower venous and pulmonary pressure. Regardless, such velocity changes would be expected to reduce intraventricular blood flow and thus create intraventricular pressure changes that could sustain the foramen opening, resulting in a VSD (Hogers et al., 1999; Hove et al., 2003; Sedmera et al., 1999). Unfortunately, attempts to catheterize these embryos and quantify intraventricular pressures were unsuccessful. Taken together, these findings suggest that TCE exposure during HH15 or HH17 (Drake et al., 2006a,b) disrupts cardiomyocyte calcium handing (Caldwell et al., 2008; Hoffmann et al., 1994) and cardiac contractility (Drake et al., 2006b), resulting in the muscular VSDs observed here.
Newly hatched chicks had largely normal cardiac function apart from an increased heart rate that would increase cardiac output and compensate for the VSD. These chicks represent the survivors, 37% of which had VSDs, among a population in which 30–50% died at embryonic stages. Thus, the hatch data likely underreport the severity and frequency of cardiac problems in this population as a whole. Endorsing this interpretation is the identification of a subset of TCE-exposed hearts prior to the mortality period that had significant deviations in heart structure and function. Because the hatched chicks were young, they had not experienced cardiac stress. Although the heart can adapt to stressors such as pressure overload, ischemia, and hypoxia, if the stressor remains unresolved, the heart reaches a limit in its ability to compensate and transitions into hypertrophy and subsequently dilatative failure. The TCE-associated pathologies seen at ED5.5 and the high prehatch mortality suggest that the least adapted chicks died prior to hatch. Additional assessments are necessary before conclusions can be drawn about the adequacy of cardiac performance in the hatched survivors.
Nonmonotonic Dose Response
In chicks, nanomolar TCE exposures cause a nonmonotonic dose response with respect to mortality as an end point, a finding consistent with our previous work documenting a nonmonotonic response for mortality and AV canal cushion cellularity (Drake et al., 2006b). Loeber et al. (1988) also reported a nonmonotonic dose response for TCE-exposed chicks with respect to heart defects. Nonmonotonic dose responses are documented for numerous chemical agents, including alcohol, opiates, formaldehyde, and serotonin (Calabrese, 2001; Calabrese and Baldwin, 2003; Gaylor et al., 2004; Sari and Zhou, 2003). Numerous mechanisms can produce a nonmonotonic response, including differing affinities for multiple enzymatic or receptor targets of the toxicant, concentration-dependent generation of metabolites having distinct effects, and concentration-dependent induction of alternative disposal pathways. The explanation for TCE’s nonmonotonic effect awaits a clearer understanding of its mechanism and identification of the proximal teratogen.
In conclusion, our data independently confirm that TCE is a cardiac teratogen at exposures in the range of the EPAs MCL. This conclusion has been controversial because heart defects have not been consistently observed following developmental exposure (e.g., Carney et al., 2006; Dorfmueller et al., 1979; Fisher et al., 2001; Healy et al., 1982; Schwetz et al., 1975). We suggest that this is due, in part, because prior studies utilized static measures, such as histology and freehand dissection (Wilson, 1965), and likely missed functional changes as well as subtle physical changes having significant functional consequences, such as those reported here. The adoption of real-time imaging methods will facilitate the detection of dysmorphologies and dysfunctions in cardiac toxicology studies.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (R01 ES11738 to J.L. and S.M.S., R37 AA11085 to S.M.S., and T32 ES007015 to E.S.R.).
Supplementary Material
References
- Agency for Toxic Substances and Disease Registry. Volatile Compounds in the Drinking Water and Adverse Pregnancy Outcomes. U.S. Marine Corps Base, Camp Lejeune, North Carolina. PB98-156540. Springfield, VA: National Technical Information Service; 1998. [Google Scholar]
- Board on Environmental Studies and Toxicology (BEST) Assessing the Human Health Risks of Trichloroethylene: Key Scientific Issues. Washington, DC: National Academies Press; 2006. [Google Scholar]
- Bove F, Shim Y, Zeitz P. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ. Health. Perspect. 2002;110:61–74. doi: 10.1289/ehp.02110s161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE. Public drinking water contamination and birth outcomes. Am. J. Epidemiol. 1995;141:850–862. doi: 10.1093/oxfordjournals.aje.a117521. [DOI] [PubMed] [Google Scholar]
- Boyer AS, Finch WT, Runyan RB. Trichloroethylene inhibits development of embryonic heart valve precursors in vitro. Toxicol. Sci. 2000;53:109–117. doi: 10.1093/toxsci/53.1.109. [DOI] [PubMed] [Google Scholar]
- Bross G, DiFranceisco D, Desmond ME. The effect of low dosages of trichloroethylene on chick development. Toxicology. 1983;28:283–294. doi: 10.1016/0300-483x(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Opiates: biphasic dose responses. Crit. Rev. Toxicol. 2001;31:585–604. doi: 10.1080/20014091111848. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Ethanol and hormesis. Crit. Rev. Toxicol. 2003;33:407–424. doi: 10.1080/713611043. [DOI] [PubMed] [Google Scholar]
- Caldwell PT, Thorne PA, Johnson PD, Boitano S, Runyan RB, Selmin O. Trichloroethylene disrupts cardiac gene expression and calcium homeostasis in rat myocytes. Toxicol. Sci. 2008;104:135–143. doi: 10.1093/toxsci/kfn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney EW, Thorsrud BA, Dugard PH, Zablotny CL. Developmental toxicity studies in crl:CD (SD) rats following inhalation exposure to trichloroethylene and perchloroethylene. Birth. Defects. Res. B. 2006;77:405–412. doi: 10.1002/bdrb.20091. [DOI] [PubMed] [Google Scholar]
- Collier JM, Selmin O, Johnson PD, Runyan RB. Trichloroethylene effects on gene expression during cardiac development. Birth. Defects. Res. A. 2003;67:488–495. doi: 10.1002/bdra.10073. [DOI] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson BV, Johnson PD, Goldberg SJ, Ulreich JB. Cardiac teratogenesis of halogenated hydrocarbon-contaminated drinking water. J. Am. Coll. Cardiol. 1993;21:1466–1472. doi: 10.1016/0735-1097(93)90325-u. [DOI] [PubMed] [Google Scholar]
- deAlmeida A, McQuinn T, Sedmera D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ. Res. 2007;100:1363–1370. doi: 10.1161/01.RES.0000266606.88463.cb. [DOI] [PubMed] [Google Scholar]
- Dorfmueller MA, Henne SP, York RG, Bornschein RL, Manson JM. Evaluation of teratogenicity and behavioral toxicity with inhalation exposure of maternal rats to trichloroethylene. Toxicology. 1979;14:153–166. doi: 10.1016/0300-483x(79)90061-1. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Hu N, Smith SM, Lough J. Cardiogenic effects of trichloroethylene and trichloroacetic acid following exposure during heart specification of avian development. Toxicol. Sci. 2006a;94:153–162. doi: 10.1093/toxsci/kfl083. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough J, Hu N, Smith SM. Trichloroethylene exposure during cardiac valvuloseptal morphogenesis alters cushion formation and cardiac hemodynamics in the avian embryo. Environ. Health Perspect. 2006b;114:842–847. doi: 10.1289/ehp.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovaara E, Hemminki K, Vainio H. Effects of methylene chloride, trichloroethane, trichoroethylene, tetrachloroethylene and toluene on the development of chick embryos. Toxicology. 1979;12:111–119. doi: 10.1016/0300-483x(79)90037-4. [DOI] [PubMed] [Google Scholar]
- Fisher JW, Channel SR, Eggers JS, Johnson PD, MacMahon KL, Goodyear CD, Sudberry DA, Warren JR, Latendress JR, Graeter LJ. Trichloroethylene, trichloroacetic acid, and dichloroacetic acid: do they affect fetal rat heart development? Int. J. Toxicol. 2001;20:257–267. doi: 10.1080/109158101753252992. [DOI] [PubMed] [Google Scholar]
- Gaylor DW, Lutz WK, Conolly RB. Statistical analysis of nonmonotonic dose-response relationships: research design and analysis of nasal cell proliferation in rats exposed to formaldehyde. Toxicol. Sci. 2004;77:158–164. doi: 10.1093/toxsci/kfh008. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Lebowitz MD, Graver EJ, Hicks S. An association of human congenital cardiac malformations and drinking water contaminants. J. Am. Coll. Cardiol. 1990;16:155–164. doi: 10.1016/0735-1097(90)90473-3. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ. Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- Healy TE, Poole TR, Hopper A. Rat fetal development and maternal exposure to trichloroethylene 100 ppm. Br. J. Anaesth. 1982;54:337–341. doi: 10.1093/bja/54.3.337. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Heinroth K, Richards D, Plews P, Toraason M. Depression of calcium dynamics in cardiac myocytes – a common mechanism of halogenated hydrocarbon anesthetics and solvents. J. Mol. Cell. Cardiol. 1994;26:579–589. doi: 10.1006/jmcc.1994.1070. [DOI] [PubMed] [Google Scholar]
- Hogers B, DeRuiter MC, Gitternberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc. Res. 1999;41:87–99. doi: 10.1016/s0008-6363(98)00218-1. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Hoshijima Y, Chien KR. Toward biologically targeted therapy of calcium cycling defects in heart failure. Physiology. 2008;23:6–16. doi: 10.1152/physiol.00033.2007. [DOI] [PubMed] [Google Scholar]
- Johnson PD, Dawson BV, Goldberg SJ. Cardiac teratogenicity of trichloroethylene metabolites. J. Am. Coll. Cardiol. 1998;32:540–545. doi: 10.1016/s0735-1097(98)00232-0. [DOI] [PubMed] [Google Scholar]
- Johnson PD, Goldberg SJ, Mays MZ, Dawson BV. Threshold of trichloroethylene contamination in maternal drinking waters affecting fetal heart development in the rat. Environ. Health. Perspect. 2003;111:289–292. doi: 10.1289/ehp.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BB, Hu N, Serrino PJ, Clark EB. Ventricular pressure-area loop characteristics in the stage 16 to 24 chick embryo. Circ. Res. 1991;68:226–231. doi: 10.1161/01.res.68.1.226. [DOI] [PubMed] [Google Scholar]
- Lagakos SW, Wessen BJ, Zelen M. An analysis of contaminated well water and health effects in Woburn, Massachusetts. J. Am. Stat. Assoc. 1986;81:583–596. [Google Scholar]
- Loeber CP, Hendrix MJ, Diez De Pinos S, Goldberg SJ. Trichloroethylene: a cardiac teratogen in developing chick embryos. Pediatr. Res. 1988;24:740–744. doi: 10.1203/00006450-198812000-00018. [DOI] [PubMed] [Google Scholar]
- McQuinn TC, Bratoeva M, Dealmeida A, Remond M, Thompson RP, Sedmera D. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev. Dyn. 2007;236:3503–3513. doi: 10.1002/dvdy.21357. [DOI] [PubMed] [Google Scholar]
- Mishima N, Hoffman S, Hill EG, Krug EL. Chick embryos exposed to trichloroethylene in an ex ovo culture model show selective defects in early endocardial cushion tissue formation. Birth. Defects. Res. A. 2006;76:517–527. doi: 10.1002/bdra.20283. [DOI] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int. Rev. Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Porter GA, Makuck RF, Rivkees SA. Intracellular calcium plays an essential role in cardiac development. Dev. Dyn. 2003;227:280–290. doi: 10.1002/dvdy.10307. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou F. Serotonin and its transporter on proliferation of fetal heart cells. Int. J. Dev. Neurosci. 2003;21:417–424. doi: 10.1016/j.ijdevneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Schwetz BA, Leong KJ, Gehring PJ. The effect of maternally inhaled trichloroethylene, perchloroethylene, methyl chloroform, and methylene chloride on embryonal and fetal development in mice and rats. Toxicol. Appl. Pharmacol. 1975;32:84–96. doi: 10.1016/0041-008x(75)90197-0. [DOI] [PubMed] [Google Scholar]
- Sedmera D, McQuinn T. Embryogenesis of the heart muscle. Heart. Fail. Clin. 2008;4:235–245. doi: 10.1016/j.hfc.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Smith MK, Randall JL, Read EJ, Stober JA. Teratogenic activity of trichloroacetic acid in the rat. Teratology. 1989;40:445–451. doi: 10.1002/tera.1420400506. [DOI] [PubMed] [Google Scholar]
- Wilson JG. Methods for administering agents and detecting malformations in experimental animals. In: Wilson JG, Warkany J, editors. Teratology Principles and Techniques. Chicago, IL: University of Chicago Press; 1965. [Google Scholar]
- World Health Organization. Guidelines for Drinking-water Quality. 3rd ed. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- Yauck JS, Malloy ME, Blair K, Simpson PM, McCarver DG. Proximity of residence to trichloroethylene-emitting sites and increased risk of offspring congenital heart defects among older women. Birth. Defects. Res. A. 2004;70:808–814. doi: 10.1002/bdra.20060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.