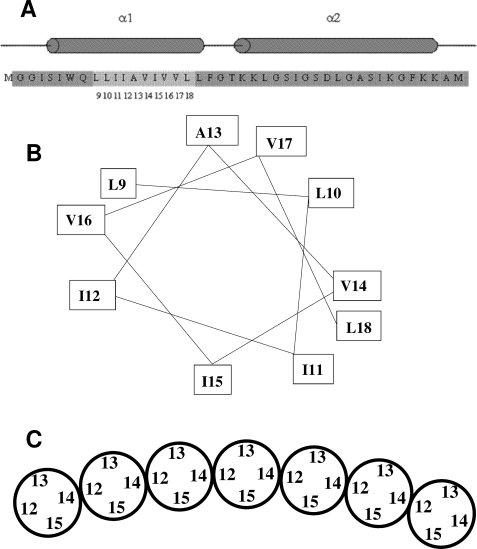
FIGURE 5.
Model for the arrangement of the transmembrane helices in the TatA complex. A, sequence and predicted secondary structure of the amino-terminal portion of E. coli TatA. Residues 1–43 of the 89 amino acid E. coli TatA protein are shown. The predicted α-helical regions of the protein are indicated by cylinders above the sequence. The numbered, lighter gray region indicates the sites where residues were substituted with cysteines and spin-labeled in this study. B, helical wheel representation of the sites within the TatA transmembrane helix to which spin labels were attached in this work. C, model for the arrangement of TatA transmembrane helices within the TatA complex derived from the spin labeling data presented in this work. The helices are shown schematically and end-on. Only a section of a TatA oligomer is shown. It is envisaged that the ends of the oligomer are extended by further subunits to form a ring. Note that the depicted organization with residue 15 facing the interior of the ring and residue 13 the outside of the ring is arbitrary. The opposite orientation is equally plausible.