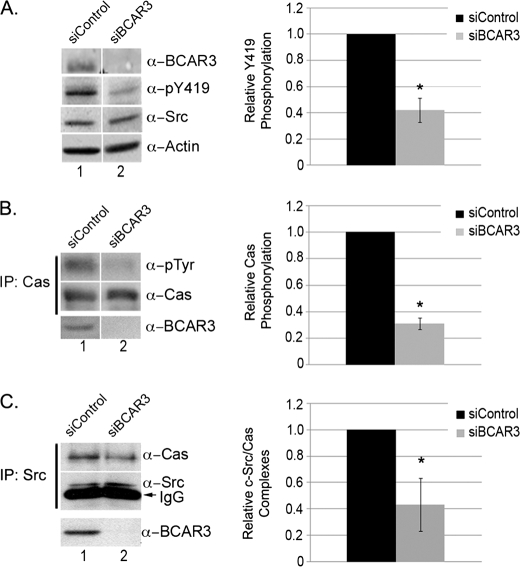
FIGURE 3.
Depletion of BCAR3 in BT549 breast cancer cells disrupts c-Src activation, Cas phosphorylation, and Cas/c-Src interactions. A, BT549 cells depleted for BCAR3 have decreased c-Src activity. BT549 breast cancer cells were transfected with nontargeting or BCAR3-specific siRNA duplexes and grown for 48 h. Whole cell lysate was immunoblotted with the indicated antibodies. The mean relative Tyr(P)-419 (α-pY419) phosphorylation was determined from four independent experiments. B, BT549 cells depleted for BCAR3 have decreased Cas phosphorylation. Cas immune complexes were generated from 72-h siRNA-treated BT549 cells and immunoblotted with antibodies specific to Tyr(P) and Cas (top two panels). Cell lysate was immunoblotted for BCAR3 to verify loss of BCAR3 expression (bottom panel). The mean relative Cas phosphorylation was determined from four independent experiments. C, BT549 cells depleted for BCAR3 have decreased Cas/c-Src interactions. c-Src immune complexes were generated from 72-h siRNA-treated BT549 cells and immunoblotted with Cas and c-Src antibodies (top panel). Whole cell lysate was also immunoblotted with BCAR3 antibodies (bottom panel). The mean Cas levels in Cas-c-Src immune complexes were determined from five independent experiments. Two-tailed Student's t tests were conducted for comparison between siControl and siBCAR3 samples for A–C. Bars indicate standard deviation; asterisk indicates significant difference from the mean at ≥95% confidence interval. Within all panels, exposure time is the same; the white vertical lines denote noncontiguous sample lanes.