FIGURE 4.
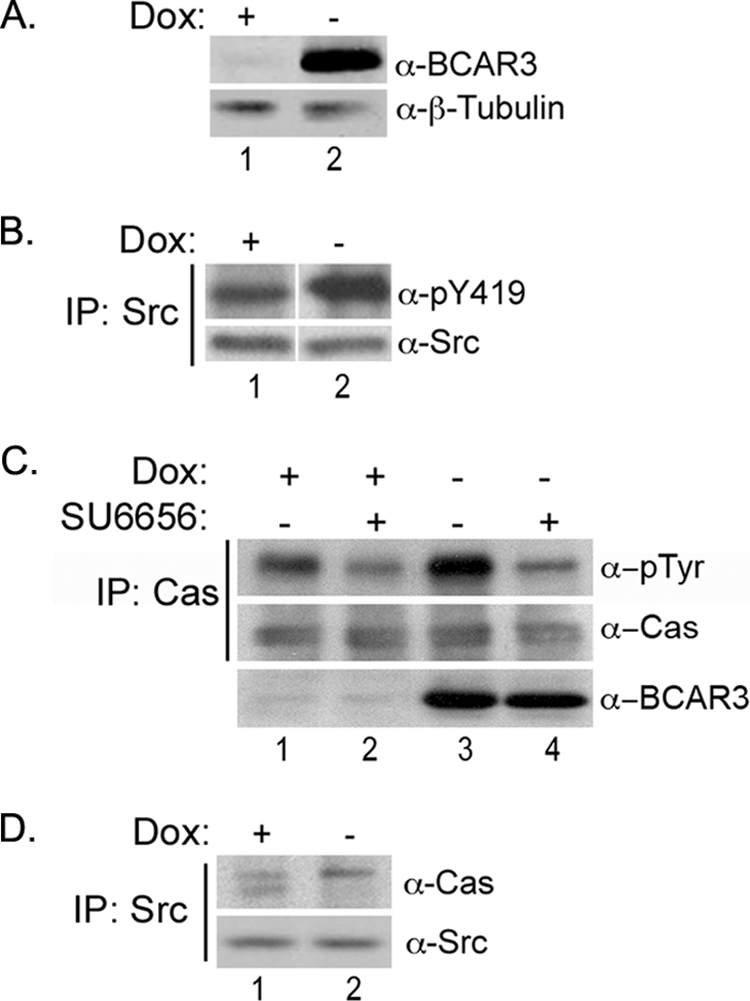
BCAR3 overexpression in MCF7 breast cancer cells increases c-Src activity and Cas phosphorylation. A, Dox-regulated expression of BCAR3 in Tet-Off MCF7 cells. MCF7 cells overexpressing BCAR3 under control of a tetracycline-responsive promoter were grown in the presence or absence of 2 μg/ml Dox for 72 h. Whole cell lysate was immunoblotted with antibodies specific to BCAR3 and β-tubulin. B, BCAR3 overexpressing cells exhibit increased c-Src activation. c-Src immune complexes were generated from MCF7 cells grown in the presence or absence of 2 μg/ml Dox for 72 h and immunoblotted with antibodies specific for Tyr(P)-419 and total c-Src. Tyr(P)-419 was increased by an average of 1.9 ± 0.43-fold in three-independent experiments (p = 0.02). The white vertical line denotes noncontiguous sample lanes; the exposure times for the +/− Dox samples in each immunoblot is the same. C, BCAR3-dependent Cas phosphorylation is mediated by Src family kinases. MCF7-B6 cells grown in the presence or absence of Dox for 48 h were treated with vehicle (DMSO) or 10 μm SU6656 and grown for an additional 24 h. Cas immune complexes were generated from lysate and immunoblotted with antibodies specific to Tyr(P) and Cas (top two panels). Whole cell lysate was also immunoblotted with BCAR3 antibodies (bottom panel). Cas phosphorylation increased by an average of 2.9 ± 0.64-fold (p = 0.009) in the presence of overexpressed BCAR3. Data shown are representative of four independent experiments. D, BCAR3 expression regulates Cas/c-Src interactions. c-Src immune complexes were generated from MCF7 cells grown in the presence or absence of 2 μg/ml Dox for 72 h and immunoblotted with Cas- and c-Src-specific antibodies.
