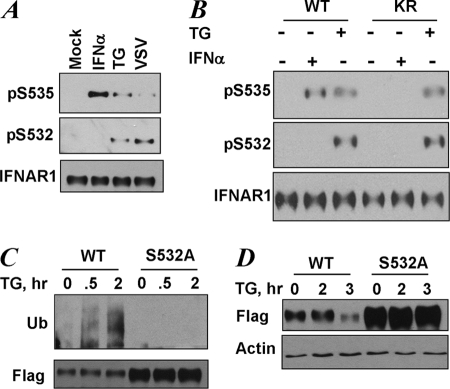
FIGURE 3.
UPR induces phosphorylation of the priming site that is required for increased ubiquitination and degradation of IFNAR1. A, endogenous IFNAR1 proteins immunopurified from HeLa cells that were untreated (Mock) or were treated with TG (1 μm for 30 min), IFNα (6000 IU/ml for 30 min), or infected with VSV (0.1 multiplicity of infection for 1 h followed by an additional 10 h of incubation in virus-free medium) as indicated, were analyzed by immunoblotting using the indicated antibodies. B, endogenous IFNAR1 proteins immunopurified from 11.1-Tyk2-null derivative cell lines reconstituted with wild type Tyk2 (WT) or catalytically deficient Tyk2 (KR) were treated with TG or IFNα (in doses indicated in A) for 30 min. C, FLAG-IFNAR1 proteins (wild type or S532A mutant) were expressed in 293T cells. The cells were pretreated with a lysosomal inhibitor (methylamine HCl, 10 mm) for 1 h to prevent degradation of ubiquitinated receptors. Then the cells were treated with TG (1 μm for the indicated times), and FLAG-IFNAR1 proteins were immunopurified under denaturing conditions and analyzed by immunoblotting using antibodies against ubiquitin (Ub, upper panel) and FLAG (lower panel). D, down-regulation of the levels of FLAG-IFNAR1 (wild type or S532A mutant) expressed in 293T cells treated with TG (1 μm for indicated times) was analyzed by immunoblotting using FLAG antibody. Comparable loading was verified by anti-β-actin immunoblot (lower panel).