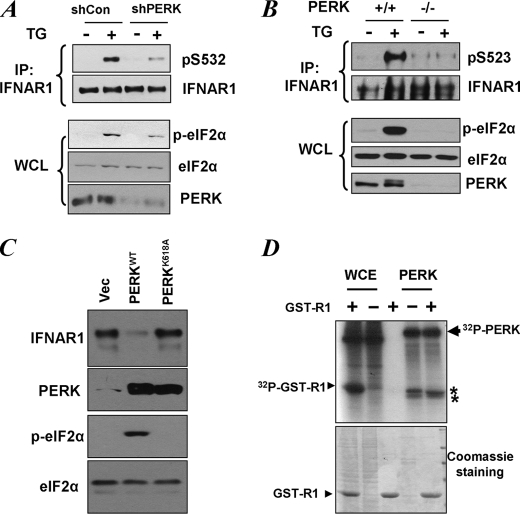
FIGURE 4.
Role of PERK in UPR-induced phosphorylation of priming site of IFNAR1. A, HeLa cells transfected with small hairpin RNA against PERK or against GFP (shCon) were treated with TG (1 μm for 30 min), and endogenous IFNAR1 proteins were immunopurified (IP) and analyzed for phosphorylation on the priming site and for total levels by immunoblotting using the indicated antibodies. Phosphorylation of a known PERK substrate eIF2α (as well as its total levels) and the levels of PERK itself were also determined in whole cell lysates (WCL). B, mouse embryo fibroblasts from wild type or PERK knock-out animals were treated with TG as indicated. The levels and priming phosphorylation of endogenous murine IFNAR1 on Ser523 (analogue of human Ser532) were analyzed by immunoblotting using indicated antibodies. The phosphorylation and levels of eIF2α and the levels of PERK in whole cell lysates were also determined. C, levels of endogenous IFNAR1 in 293T cells transfected with wild type or the catalytically deficient mutant (K618A) of PERK were analyzed by immunoprecipitation followed by immunoblotting using an anti-IFNAR1 antibody. The levels of PERK, phosphorylated PERK, and levels of eIF2α were also examined. D, whole cell extracts (WCE) from 293T cells or recombinant bacterially produced constitutively active ΔN-PERK were incubated alone or with GST-IFNAR1 in the presence of radiolabeled [γ-32P]ATP as indicated. Resulting phosphorylation of GST-IFNAR1 or contaminants and autophosphorylation of PERK was determined by SDS-PAGE followed by Coomassie staining and autoradiography. The positions of PERK, GST-IFNAR1, and some irrelevant contaminants (denoted by asterisks) are indicated. Vec, empty vector.