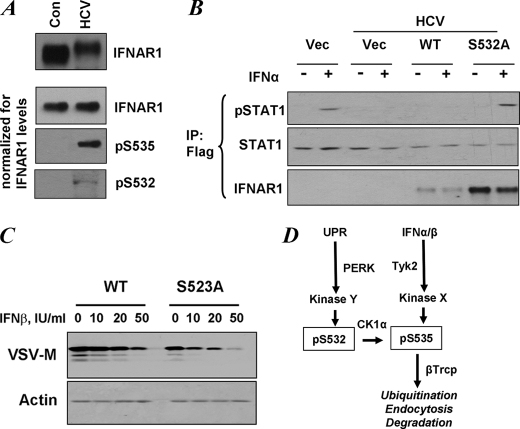
FIGURE 5.
Priming phosphorylation of IFNAR1 contributes to regulation of the extent of IFNα/β signaling. A, control (Con) human Huh7 cells and those expressing the HCV replicon were analyzed for IFNAR1 levels by immunoprecipitation-immunoblotting (upper panel). The lower three panels depict the experiments where gel loading was normalized to achieve comparable levels of immunopurified IFNAR1 in each lane. Phosphorylation of IFNAR1 on Ser532 and Ser535 was determined by immunoblotting using the indicated antibodies. B, control human Huh7 cells and those expressing the HCV replicon were transfected with FLAG-tagged STAT1 alone with empty vector (Vec) or FLAG-IFNAR1 (wild type (WT) or S532A mutant) and were untreated or treated with IFNα (60 IU/ml for 30 min) as indicated. Lysates of these cells were immunoprecipitated (IP) using anti-FLAG antibody and analyzed by immunoblotting using antibodies against phospho-STAT1, total STAT1, and IFNAR1. C, mouse embryo fibroblasts from IFNAR1-null animals were reconstituted with murine FLAG-IFNAR1 (wild type or S523A mutant, which is a mouse analogue of human S532A mutant). The cells were treated with indicated doses of murine IFNβ for 1 h, incubated for 8 h in fresh medium, and then infected with VSV (multiplicity of infection of 0.1). Expression of VSV-M protein was analyzed 16 h later by immunoblotting. Levels of β-actin were also determined (lower panel). D, model for ligand-dependent and ligand-independent ubiquitination and degradation of IFNAR1. Both pathways converge at the level of degron phosphorylation (Ser(P)535, pS535). Signaling induced by IFN and dependent on the activity of Tyk2 does not require either CK1α (24) or priming phosphorylation (this study). Ligand-independent pathway initiated by inducers of UPR does not need either ligand or Tyk2 activity but requires CK1α (26) and PERK-dependent priming phosphorylation (this study).