FIGURE 7.
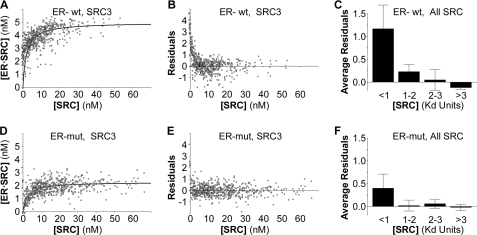
A high affinity component of the ER·SRC-RID complex is dependent on the ERα dimer interface. A, the binding curve and B, residuals from that binding curve for the interaction of wild-type (wt) ERα with wild-type SRC3-RID show a strong tendency for the data points to lie above the curve at very low SRC-RID concentrations. mut, mutant. C, the residuals for data points at low SRC-RID concentrations (<1 Kd) are elevated consistently for six total studies (mean ± S.D. of the average residuals in each of two studies each for the SRC-1, -2, and -3 RIDs). D–F, analysis of SRC-RID interaction with the monomeric ERα mutant collected in parallel suggests that the high affinity component of ER·SRC-RID interaction is dependent upon an ability to form a dimer.