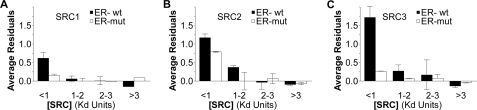
FIGURE 8.
High affinity ERα complexes with the SRC1, -2, or -3 RIDs. Analysis of the extent to which the residuals deviate from the best-fitting curve for ERα interaction with A, SRC1-RID, B, SRC2-RID, and C, SRC3-RID. Interactions were measured with the wild-type (wt, filled bars) or monomeric ERα mutant (mut, open bars). Data represent the mean ± range of the average residuals in two independent studies each for the SRC-1, -2 and -3 RIDs.