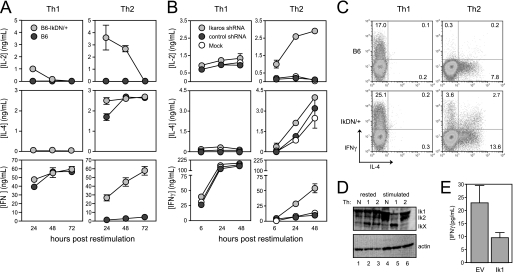
FIGURE 1.
Ikaros DNA binding activity is required for cytokine polarization during T helper development. A, CD8-depleted spleen cells from wild-type (dark gray circles) and IkDN/+ (light gray circles) mice were stimulated with soluble anti-CD3/28 Abs with the addition of IL-12 and anti-IL-4 Ab for Th1 or IL-4, anti-IFNγ, and anti-IL-12 for Th2. B, wild-type CD8-depleted spleen cells were stimulated under either Th1 or Th2 conditions (as in A above) and transduced with retroviral vector encoding Ik1 shRNA (light gray circles), control scrambled shRNA (dark gray circles), or mock-transduced (white circles). For both A and B, cultures were harvested, washed, and restimulated with plate-bound anti-CD3/28 Ab. Secretion of IL-2, IL-4, and IFNγ secretion was measured by ELISA. C, three days following restimulation, wild-type (top panels) and IkDN/+ (bottom panels) cultures were boosted by the addition of PMA and ionomycin in the presence of monensin for 5 h, and cells were stained for surface CD4 and intracellular IL-4 (x axis) and IFNγ (y axis). Plots are gated on CD4+ cells, and the percentage of CD4+ cells that are positive for one or both cytokines is shown. D, wild-type neutral (lanes 1 and 4), Th1 (lanes 2 and 5), and Th2 (lanes 3 and 6) cells were generated as in A and rested or restimulated with plate-bound anti-CD3/28 Ab for 18 h, and Ikaros expression was assessed by immunoblotting using an Ab against the N terminus. E, purified CD4+ T cells stimulated under neutral conditions were transduced with empty MIGR1 retroviral vector (EV) or MIGR1 encoding full-length Ikaros (Ik1). Cells were rested and restimulated for 24 h with plate-bound anti-CD3/28 Ab, and IFNγ secretion was measured by ELISA. The data shown are representative of three independent experiments. Error bars are S.E. for biological replicate cultures.