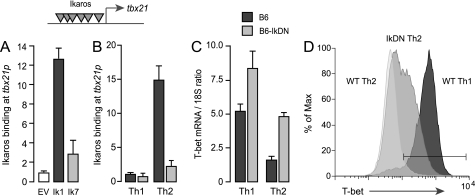
FIGURE 2.
Ikaros is a direct transcriptional repressor of the endogenous tbx21 gene. A, Ikarosnull JE131 cells were transduced with empty MIGR1 retroviral vector or reconstituted with MIGR1 encoding FLAG-tagged Ikaros lacking the DNA binding domain (Ik7) or full-length, FLAG-tagged Ikaros (Ik1). Chromatin extracts were precipitated with Ab against the FLAG epitope, and precipitated DNA was probed for the tbx21 promoter by qRT-PCR. Ikaros binding was calculated as the ratio of the specific Ab ChIP signal over the background isotype Ab control ChIP signal. Gray inverted triangles indicate putative Ikaros binding elements within the tbx21 promoter. B, wild-type (dark gray bars) and Ik7DN/+ (light gray bars) CD4+ T cells were polarized under Th1 or Th2 conditions for 24 h, and chromatin extracts were precipitated with Ab against native Ikaros. Precipitated DNA was probed for the tbx21 promoter by qRT-PCR. C and D, wild-type (dark gray bars) and Ik7DN/+ (light gray bars) Th1 and Th2 cells were restimulated on plate-bound CD3/28 Ab for 3 days. RNA was isolated, and expression of T-bet mRNA was measured by qRT-PCR (C), and T-bet protein expression in the CD4+ subset was detected by flow cytometry using fluorochrome-conjugated Ab against T-bet (D). The white histogram depicts T-bet staining of wild-type Th2 cells; the light gray histogram depicts staining of IkDN Th2 cells; and the dark gray histogram depicts wild-type Th1 cells. T-bet staining in IkDN Th1 cells was comparable with wild-type Th1 cells (not shown). The gate represents the 98% confidence interval of the isotype control stain. The data depicted are representative of two separate experiments. Error bars are S.E. for biological replicate cultures.