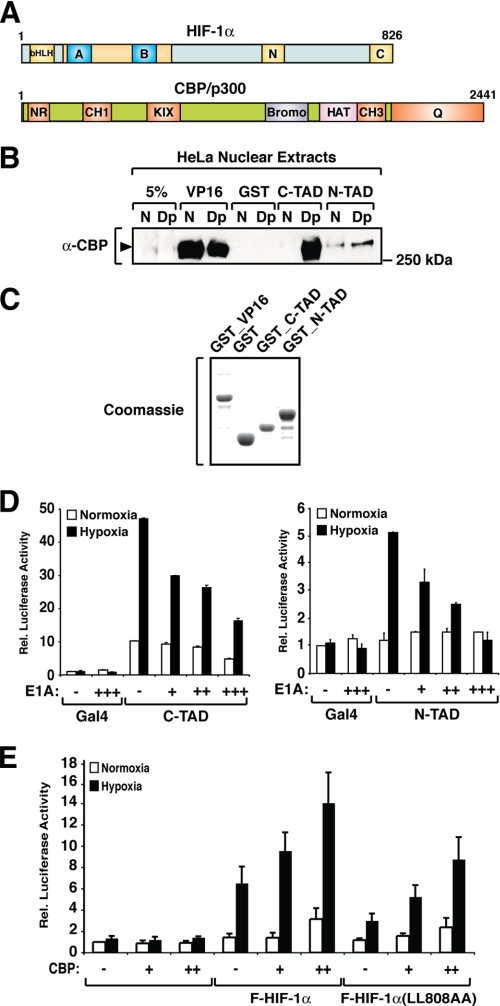
FIGURE 1.
HIF-1α N-TAD interacts with endogenous CBP. A, schematic representation of HIF-1α and CBP/p300 domains. HIF-1α contains an N-terminal DNA-binding domain followed by a helix-loop-helix dimerization interface (bHLH) and the PAS domains (blue boxes labeled A and B). The N- (N) and C-terminal (C) transactivation domains are located in the C-terminal portion of the protein. CBP/p300 contains several domains that mediate interaction with other proteins, such as the NR domain (interaction with nuclear receptors), CH1 and CH3, and the C-terminal glutamine-rich domain (Q). B, HIF-1α N-TAD interacts with endogenous CBP. Nuclear extracts from HeLa cells kept at normoxia (N) or treated with 2,2′-dipyridyl (Dp) were incubated with GST-fused VP16, C-TAD, or N-TAD. Precipitated proteins were separated by SDS-PAGE, and CBP was detected by immunoblot analysis using an anti-CBP antibody (α-CBP). C, Coomassie staining of bacterially expressed GST-fused proteins. D, N-TAD transactivation activity is inhibited by expression of E1A. HEK 293 cells were transfected with 500 ng of GAL4-driven luciferase reporter gene and 10 ng of plasmids encoding FLAG-GAL-C-TAD (left panel) or FLAG-GAL4-N-TAD (right panel) in the absence or presence of increasing concentrations of an E1A expression plasmid (10, 20, or 50 ng). The data are presented as luciferase activity relative to cells transfected with pFLAG-GAL4 and incubated at normoxia. The values represent the means ± S.E. of three independent experiments performed in duplicate. E, full-length HIF-1α mutant with a nonfunctional C-TAD is responsive to CBP. HEK 293 cells were transfected with a HRE-driven luciferase reporter plasmid and plasmids encoding FLAG-HIF-1α or FLAG-HIF-1α(L808A/L809A) (F-HIF-1α(LL808AA)) in the presence or absence of a CBP expressing plasmid. The data are presented as luciferase activity relative to cells transfected with pCMX and incubated at normoxia. The values represent the means ± S.E. of three independent experiments performed in duplicate.