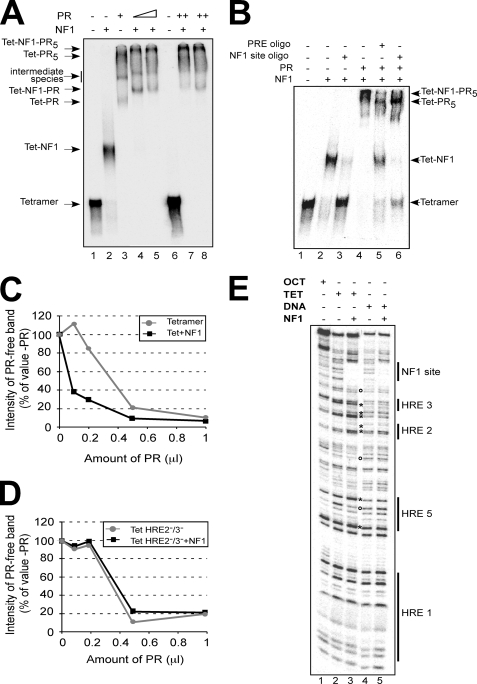
FIGURE 4.
PR and NF1 bind cooperatively to the MMTV promoter assembled on a tetramer of histones H3 and H4. A, end-labeled reconstituted MMTV tetramer particles were incubated with PR and NF1 as indicated. The established complexes were analyzed by electrophoresis and autoradiography. The identity of the main bands is indicated on the left margin. B, end-labeled reconstituted MMTV tetramer particles were incubated with PR, NF1, and 100-fold molar excess of an oligonucleotide (oligo) containing either a progesterone-responsive element (PRE) or a NF1 consensus site, as indicated. After incubation the established complexes were analyzed as described for A. The identity of the main bands is indicated on the right margin. C, end-labeled reconstituted MMTV tetramer particles were incubated with NF1 and increasing amounts of PR, and the samples were analyzed as in A. The bands corresponding to free TET and free TET-NF1 were quantified and are plotted, as percentages of the values in the absence of added PR, against the amount of added PR. D, end-labeled reconstituted HRE 2−/3− MMTV tetramer particles were incubated with NF1 and increasing amounts of PR, and the samples were analyzed and plotted as in C. E, end-labeled reconstituted MMTV octamer and tetramer particles were incubated with NF1 and digested with DNase I as previously described (34). End-labeled free MMTV DNA was used as a control (lanes 4 and 5). The asterisks and circles indicate the NF1-dependent DNase I-hypersensitive and protected sites, respectively. A representative of two independent experiments is shown.