FIGURE 8.
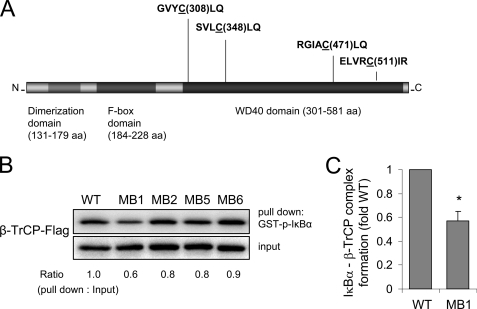
Cys-308 in β-TrCP is involved in the binding of phosphorylated IκBα. A, schematic diagram of the human β-TrCP gene showing the three primary domains: dimerization domain, F-box domain, and substrate binding WD40 domain (β-propeller region). Partial amino acid sequence of Blade 1, Blade 2, Blade 5, and Blade 6 is shown, and the cysteines targeted for mutagenesis are underlined. B, a representative Western blot (bottom) shows the concentrations of FLAG-tagged wild type β-TrCP or mutant β-TrCP with cysteine to alanine changes, specifically C308A (MB1), C348A (MB2), C478A (MB5), or C511A (MB6) transiently expressed in HEK 293 cells (input). Cell extracts were incubated with recombinant phospho-IκBα-GST and then subjected to glutathione-Sepharose precipitation (pull down). The amounts of β-TrCP associated with GST-phospho-IκBα were determined by Western blot analysis with anti-FLAG antibodies (top). Two additional experiments provided similar results. Mean optical density for IκBα associated with β-TrCP (wild type (WT)) or β-TrCP MB1 is shown in C (means ± S.D.; n = 3; *, p < 0.05). aa, amino acids; WT, wild type.