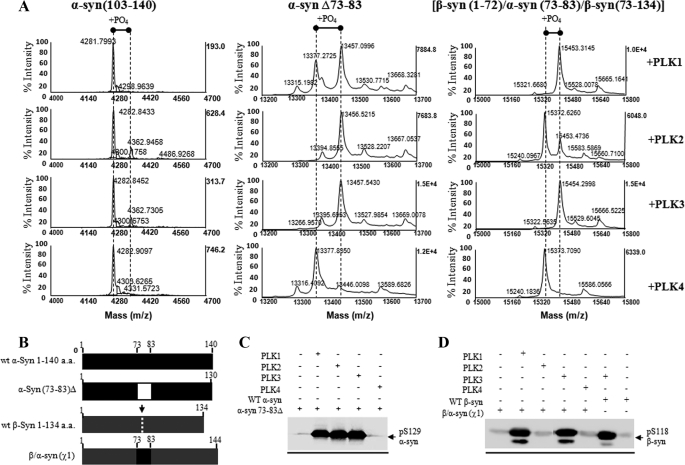
FIGURE 4.
Removal of the entire N-terminal residues (positions 1–103) abolishes Ser-129 phosphorylation, whereas deletion of the NAC residues 73–83 has no effect on Ser-129 phosphorylation by PLK1 to -3. A, MALDI-TOF analysis of the PLK-phosphorylated Δ1–103 α-syn, Δ73–83 α-syn, and chimeric β-syn(χ1) proteins. B, schematic representation of the WT α- and β-syn, deletion α-syn Δ73–83 and chimeric β-syn(χ1) proteins. C, Western blotting analysis of the in vitro phosphorylation of these constructs at Ser-129 by PLK1 to -4 using anti Ser(P)-129 antibody (1:5000; Wako). D, Western blotting analysis of the PLK-phosphorylated WT and chimeric β-syn(χ1) probed with Ser(P)-118 antibody (1:250) (1000 ng/lane). a.a., amino acids.