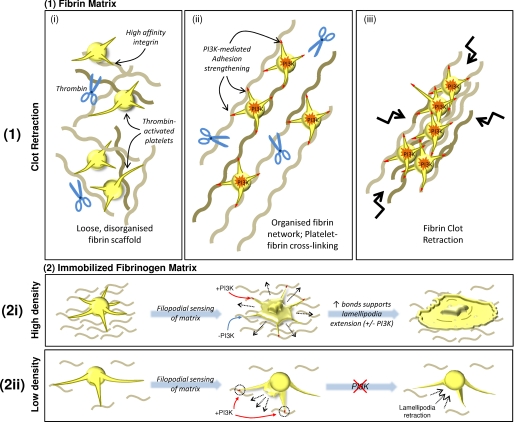
FIGURE 7.
Proposed model for PI3K-mediated regulation of adhesion strengthening and cellular retraction of fibrin polymers. 1, during early phases of platelet-mediated clot retraction, thrombin-activated platelets form adhesive contacts with sparse fibrin polymers. Although high in affinity, due to a low matrix density, these adhesive contacts are relatively unstable and inefficient in transmitting platelet contractile forces. The activation of PI3K strengthens integrin adhesion contacts (as depicted by red-tipped filopodia), potentially through integrin redistribution, enabling efficient transmission of platelet contractile forces to the fibrin clot, leading to fibrin polymer reorganization and subsequent clot retraction. 2, the requirement for PI3K-mediated adhesion strengthening is also evident in thrombin-stimulated platelets adhering to immobilized fibrinogen. In the presence of a high density fibrinogen matrix (2i), numerous high affinity integrin contacts are formed rapidly and simultaneously. The number of adhesive contacts formed with the high density matrix at any one time, both by the main platelet body and subsequently by tips of extended filopodia, provide enough adhesive support to facilitate isotropic extension of lamellipodia, even in the absence of PI3K signaling. However, under conditions of low matrix density (2ii), the main platelet body is unable to form multiple stable contacts with the matrix, resulting in transient adhesive episodes (as demonstrated in supplemental Video 1). As with platelets on a high density matrix, filopodia are extended; however, due to low matrix density, these filopodia form unstable contacts, instead requiring PI3K-mediated adhesion strengthening to stabilize adhesion, and enable directed extension of lamellipodia between adjacent filopodial extensions. In the absence of PI3K signaling, the unstable nature of these adhesion contacts results in the formation of short lamellipodia that are transiently extended and retracted into the main cell body, resulting in an inability of the platelet to fully spread.