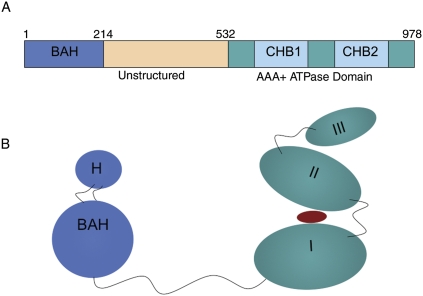
Figure 1.
Domain structure of Sir3. (A) The primary domain structure of Sir3p with important domains highlighted in different colors. The first 214 amino acids comprise the BAH domain in blue; the region from amino acids 214 to 532 (tan) is largely unstructured (McBryant et al. 2006); and from amino acids 532 to 978 (green), Sir3p is similar to the CDC6 subfamily of the AAA ATPase domain. CHB1 and CHB2 (blue) indicate the C-terminal histone-binding (CHB) activity associated with this portion of Sir3p. The BAH domain also contains histone-binding activity. (B) A cartoon version of the tertiary structure of Sir3 based on the crystal structures of the BAH domain 2FL7 (Hou et al. 2006) and the Archaeal cdc6 protein ortholog 1FNN (chain A) (Liu et al. 2000). The BAH domain is colored in blue, and the C-terminal domain is colored in teal. The red oval placed between domains I and II of the C-terminal region of Sir3 indicates a possible nucleotide moiety such as O-AADPR that could bind to Sir3p.