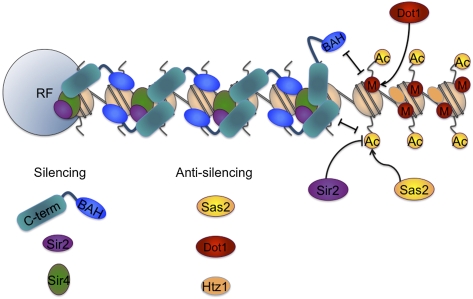
Figure 3.
Competing actions for silencing followed by reinforcements. This figure is largely based on Altaf et al. (2007) and Buchberger et al. (2008). There are silencing-promoting actions such as Sir2p deacetylating the histone tails, which creates a high-affinity site for the SIR complex. Several actions inhibit silencing: One is the inherent instability of the SIR complex, and a second is the competition between Sir2p and Sas2p for the acetylation state of histone H4 K16. HTZ1 deposition also contributes to the destabilization of silencing (Venkatasubrahmanyam et al. 2007). Acetylation of H4 K16 leads to decreased affinity of the SIR complex for the nucleosomes and especially for that of Sir3 and the nucleosome; this loss of affinity is reinforced by the competition between Dot1p and Sir3p for the methylation status of histone H3 K79. Sir3p binds the LRS surface of the nucleosome, which encompasses H3 K79 and occludes its methylation by Dot1p; conversely, when H3 K79 is methylated, Sir3p binding is blocked. The orientation of Sir3p is meant to suggest that the N-terminal BAH and the C terminus of Sir3p may bind to both the LRS domain and the histone N-terminal tails, and that this dual binding may in fact be crucial to compaction of chromatin and silencing. Additionally, the Sir3 C terminus is shown to be most sensitive to the actelyation of H4 K16, which inhibits Sir3 “lockdown” onto the chromatin (Johnson et al. 2009), and hence spreading of the silent chromatin. Sir4p is depicted as binding to linker DNA as suggested in Martino et al. (2009), but this aspect of the model should be considered preliminary. The stoichiometry of the SIR complex is also preliminary, and is based primarily on Kimura and Horikoshi (2004) and Johnson et al. (2009).