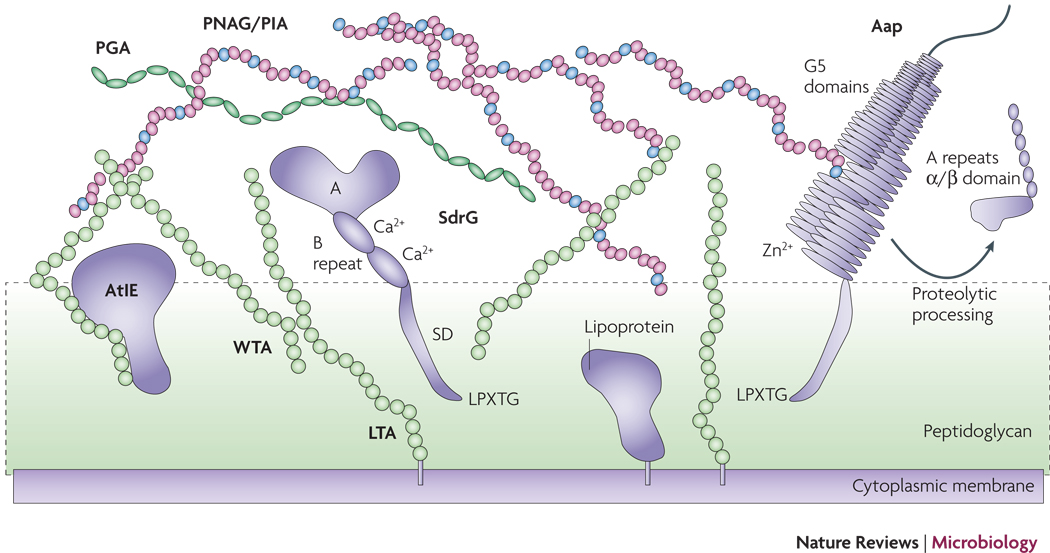
Figure 2. The S. epidermidis cell surface.
Proteins such as SdrG and Aap may be attached to the cell surface via sortase-catalyzed covalent anchoring. These proteins harbor a characteristic LPXTG motif at the C-terminus, of which the threonine residue is linked to peptidoglycan. Many autolysins such as AtlE are anchored non-covalently, likely via interaction with teichoic acids. Furthermore, lipoproteins are surface-attached via their fatty acid anchor that penetrates the cytoplasmic membrane. AtlE is a bifunctional adhesin/autolysin that contributres to biofilm formation by its surface hydrophobicity and to host matrix protein binding. SdrG is an example of the Sdr protein family of MSCRAMMs. It stretches the peptidoglycan layer by its SD repeat region and binds fibrinogen via its A region. The B repeats harbor a Ca2+ binding EF-hand domain. Aap proteins aggregate via Zn2+-dependent G5 domains and form fibrils that likely connect cells in the biofilm matrix. G5 domains also bind N-acetylglucosamine and may thus interact with the N-acetylglucosamine exopolysaccharide PNAG/PIA. PNAG/PIA is cationic and likely interacts with negatively charged surface polymers such as teichoic acids (lipoteichoic acids, LTA and wall teichoic acids, WTA) and poly-γ-glutamic acid (PGA).