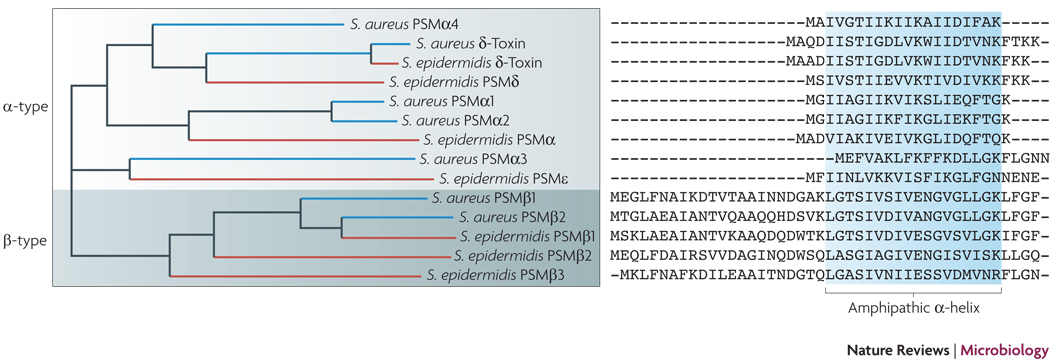
Figure 4. Phenol-soluble modulins.
Sequence alignment of S. epidermidis and S. aureus PSMs. PSMs serve as immune evasion molecules to their bacterial producer and, on the other hand, as PAMPs for pathogen recognition to the host. All PSMs contain an amphipathic α-helix and N-terminal N-formyl methionine, as they are secreted as the direct translational product without processing in an unknown manner. PSMs of the α-type are relatively short, ~ 20–25 amino acids. Particularly the S. aureus PSMα peptides 1 through 4 are strongly cytolytic. PSMs of the β-type are longer, ~ 45 amino acids, and do not have considerable cytolytic activity. Only the δ-toxin, an α-type PSM with moderate cytolytic activity, and the β-type PSMs are secreted by S. epidermidis in large amounts. Although part of the psmβ operon, the PSMβ3 peptide is not found in S. epidermidis culture filtrates for unknown reasons. The psmβ1 gene is duplicated in some strains of S. epidermidis.