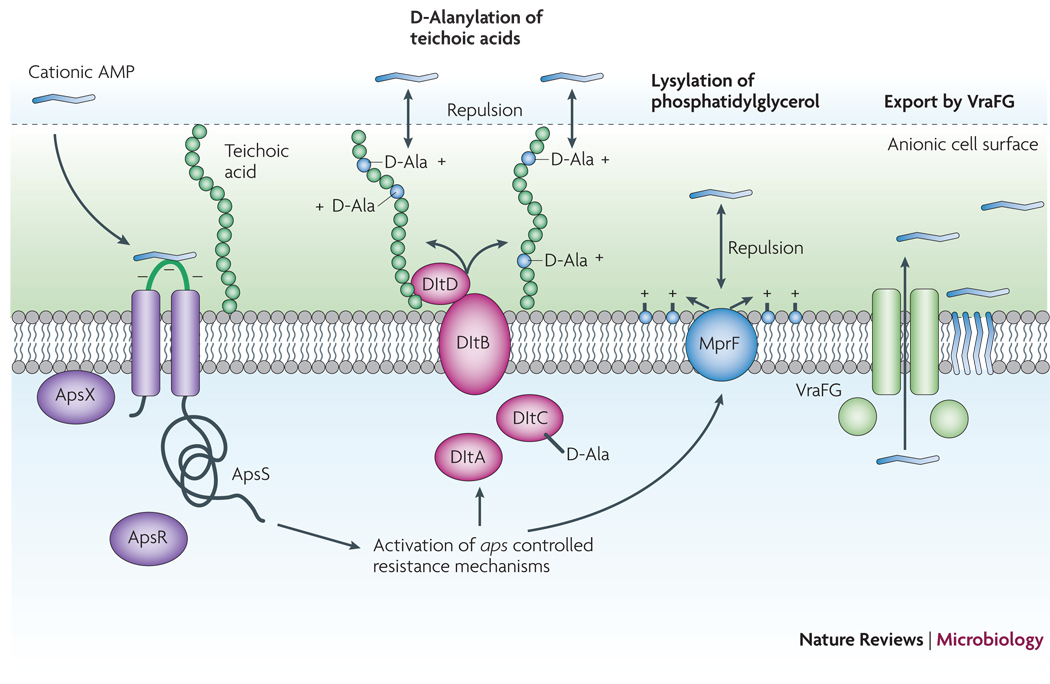
Figure 5. The Aps antimicrobial peptide sensor/regulator.
Cationic AMPs attach to the negatively charged bacterial surface and membrane by electrostatic interaction, a prerequisite for AMP antimicrobial activity, which is often based on pore formation in the bacterial cytoplasmic membrane. The S. epidermidis ApsS AMP sensor has one short extracellular loop with a high density of negatively charged amino acid residues that interacts with cationic AMPs. Transduction of this signal via ApsS and the accessory, essential ApsX, which has a yet unknown function, triggers expression of key AMP resistance mechanisms. The D-alanylation of teichoic acids, encoded by the products of the dlt operon, and lysylation of phosphatidylglycerol, catalyzed by the MprF enzyme, result in a decreased negative charge of the cell surface and membrane, respectively, leading to decreased attraction, or repulsion, of cationic AMPs. The VraFG ABC transporter also promotes resistance to AMPs and likely functions as an AMP exporter.