Abstract
AIM
Positive net work produced during cyclic contractions is partially limited by relaxation kinetics, which to date, have not been directly investigated. Therefore the purpose of this investigation was to determine the influence of relaxation kinetics on cyclic work.
METHODS
Soleus muscles of four cats were isolated and subjected to a series of work loops (0.5, 1, 1.5 and 2 Hz cycle frequencies) during which stimulation terminated prior to the end of the shortening phase to allow for complete muscle relaxation and matched discrete sinusoidal shortening contractions during which stimulation remained on until the completion of the shortening phase. Muscle length changes during these protocols were centered on optimum length and were performed across muscle lengths that represented walking gait.
RESULTS
When muscle excursions were centered on Lo relaxation kinetics decreased muscular work by 2.8 ± 0.8%, 12.1 ± 4.1%, 27.9 ± 4.5% and 40.1 ± 5.9% for 0.5, 1, 1.5, and 2 Hz respectively. However, relaxation kinetics did not influence muscular work when muscle excursions represented walking gait. In addition, muscular work produced at muscle lengths associated with walking gait was less than the work produced across Lo (55.7 ± 20.0%, 53.5 ± 21.0%, and 50.1 ± 22.0% for 0.5 Hz, 1 Hz and 1.5 Hz respectively).
CONCLUSION
These results imply that relaxation kinetics are an important factor that limit the ability of muscle to produce work; however, relaxation kinetics influence on physiological function may depend on the relation between the optimum length and natural excursion of a muscle.
Keywords: Relaxation kinetics, skeletal muscle, work
Introduction
Utilizing a variety of muscle preparations, previous investigators have described the limitations imposed on muscular work by activation and relaxation kinetics (Caiozzo and Baldwin, 1997, Marsh, 1990, Askew and Marsh, 1998, Askew et al., 1997, Stevens, 1996, Syme and Tonks, 2004, James et al., 1996). Caiozzo and Baldwin (1997) compared actual work performed during work loops to a theoretical work loop determined by Hill’s force-velocity equation and instantaneous activation and relaxation kinetics at the beginning and end of the shortening phase. They reported activation and relaxation kinetics were important factors that limit mechanical work produced by skeletal muscle particularly at higher cycle frequencies. Using a similar model, James, Young, Cox, et al. (1996) demonstrated that activation and relaxation rates limit muscular power produced during cyclic contractions. Furthermore, these limitations exert a greater influence when the muscle is fatigued (Syme and Tonks, 2004, Askew et al., 1997). Syme and Tonks (2004) investigated the effects of fatigue on muscle twitch characteristics, tetanic force and work production using the work-loop technique. They attributed a severe reduction in the muscle’s ability to perform work during cyclic contractions, compared to isometric force, to an increase in relaxation time by more than 400%.
Despite the documented importance of relaxation kinetics during cyclic contractions, to date, the limitations they impose on work have not been directly investigated. As mentioned previously, Caiozzo and Baldwin quantified the differences between actual work and theoretical work; however, the muscle lengths they utilized remained on or near the plateau of the force-length curve. There is evidence to suggest that force-length effects, and history-dependent effects (i.e., shortening induced deactivation), not included in their model, may influence relaxation kinetics and the limitations they impose on work. For example, the rate of relaxation increases as muscle length decreases (Caiozzo and Baldwin, 1997, Rassier and MacIntosh, 2002, Brown and Loeb, 2000). As a result, the limits imposed by relaxation on muscular work may vary with the muscle lengths over which the contractions are performed. Furthermore, if shortening induced deactivation (Abbott and Aubert, 1952, Herzog et al., 2000, Josephson and Stokes, 1999, Meijer, 2002, Herzog et al., 1998) is not accounted for, theoretical work would be overestimated. Therefore, a more direct approach to determining the limitations imposed by relaxation kinetics could improve our understanding of muscle’s ability to produce work.
Investigating the limitations of relaxation kinetics across a range of muscle lengths is also warranted. Although many investigators have centered work producing contractions on Lo, these muscle lengths may or may not relate to normal physiological movements. Herzog and colleagues (1992) reported the physiological range of motion for cat soleus to be on the ascending limb of the force-length curve. Likewise, Josephson and Stokes (1987) reported maximum isometric force for scaphognathite muscle L2B was achieved at muscle lengths 10–20% greater than the longest length reached in vivo. There is evidence that this difference between physiological muscle lengths and optimal muscle length is dependent on chronic use. Herzog and colleagues found that the force-length properties of the rectus femoris muscle differed systematically between runners and cyclists and suggested that this difference most likely resulted from the requirements imposed on that muscle by the different sports (Herzog et al., 1991).
The primary purpose of this investigation was to determine directly the limitations of relaxation kinetics on work. A secondary purpose was to compare the ability of muscle to perform work, and limitations of relaxation kinetics across two different muscle lengths (muscle length excursions centered on Lo and muscle length excursions representative of walking gait). We hypothesized that relaxation kinetics would significantly influence the ability of muscle to perform work during cyclic contractions at both muscle lengths. Further, we hypothesized that these limitations would be reduced when muscle length excursion represented walking gait due to increased relaxation rate and decreased force production. Finally, during walking gait cat soleus operates on the ascending limb of the force-length curve, therefore we expected the muscle’s ability to perform work would be reduced when the muscle length excursion represented walking gait compared to those centered on Lo.
Methods
All surgical and experimental protocols used in this investigation have been approved by University of Utah Institutional Animal Care and Use Committee. Four fasted cats (5.48 ± 0.78 kg) were initially anesthetized with Telezol (10 mg·kg−1). The animals were intubated and general anesthesia was maintained by isoflurane (1.5%–2.5%). ECG, HR, expired CO2, O2 saturation, body temperature, and blood pressure were monitored and recorded every 15 minutes to ensure a stable anesthetic plane and health of the animal. Lactated Ringer’s solution (600 mg sodium chloride, 310 mg sodium lactate, 30 mg potassium chloride and 20 mg calcium chloride per 100 ml) was administered intravenously at a rate of 25 ml·h−1.
Surgery involved an incision along the posterior aspect of the lower limb. The distal tendon of the soleus muscle was identified and separated from the tendons of the plantaris and gastrocnemius muscles. The calcaneous was severed leaving a bone fragment attached to the distal portion of the soleus tendon, which was connected to a load cell (Honeywell model 31, Morristown, NJ), servo-tube (Copley Controls STA25, Canton, MA) and linear encoder (Celesco, PT1E Chatwsorth, CA). During the experiments, position was controlled by the servo-tube, and force and position were simultaneously recorded by the force transducer and linear encoder (4000 Hz). The experimental animal was rigidly fixed to the table using bone pins placed in the femur and tibia. The muscle was kept moist and warm (32 °C) during the experiment through the application of mineral oil and a heat lamp.
The muscle was maximally stimulated (70Hz, 500µsec pulse width) using a Grass SD9 stimulator via 10×10 Utah Slanted Electrode Array (USEA)(Branner et al., 2001). The USEA was positioned on the sciatic nerve from the lateral side of the animal 2–4 cm proximal to the nerve’s branching into tibial and fibular nerves. A pneumatic impulse insertion technique was used to insert the array (Rousche and Normann, 1992). The electrodes on the array were then mapped (as described elsewhere (Branner et al., 2001)) to determine which electrodes stimulated motor neurons that innervated the soleus. The single electrode in which the least amount of voltage required to elicit a maximal contraction of soleus was used for the experimental protocols.
All muscle length excursions (oscillating change in muscle length) used in this experiment were based on muscle length changes produced during walking gait (Goslow et al., 1973, Gregor et al., 2006, Carlson-Kuhta et al., 1998). As a result, the absolute muscle length change used in this investigation varied between cats. More specifically, larger cats were subjected to larger muscle length excursions, but equal muscle strain (approximately 12% based on previous literature (Goslow et al., 1973)). Prior to cutting the calcaneous, a pin was inserted into the posterior side of the tibia and 9-0 suture was tied to the tendon. Distances between the bone pin and tendon suture were measured through a range of ankle joint angles defined as the included angle between the shank and dorsal surface of the foot. Relating the distance between the bone pin and tendon suture to ankle angle allowed for the identification of the muscle lengths associated with cat walking gait (85–130°) (Goslow et al., 1973, Gregor et al., 2006, Carlson-Kuhta et al., 1998) after the distal tendon was detached from the limb. Following these initial measures, the calcaneous was severed and the tendon was connected to the force transducer. Lo was determined utilizing a series of isometric contractions (400 ms pulse train) in which the initial muscle length was associated with 130° ankle angle and muscle length increased in 2 mm increments with each successive isometric contraction.
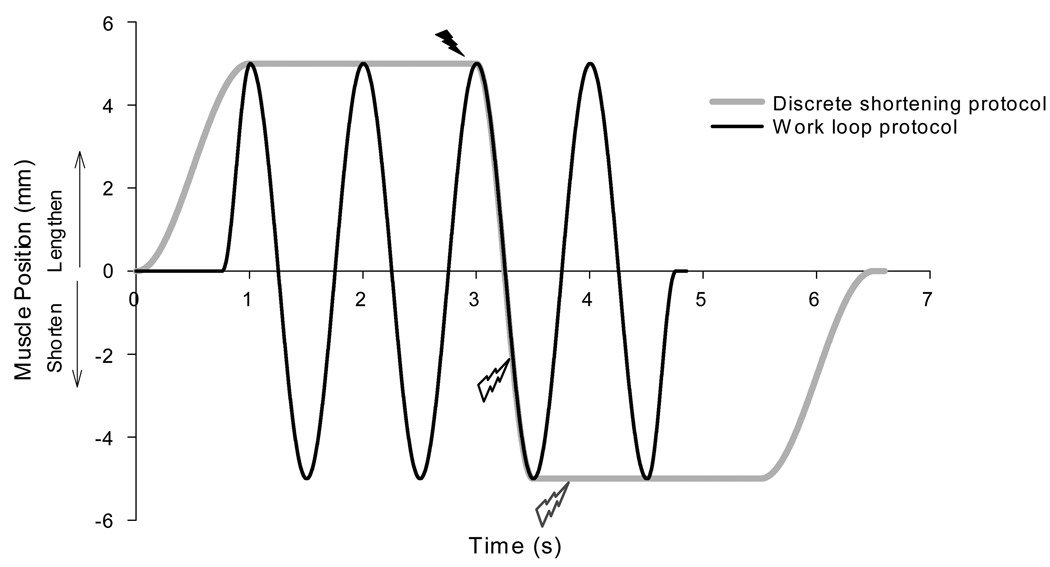
For each experimental animal, the soleus muscle was subjected to a series of four work loops and four discrete sinusoidal shortening contractions (an example of a single work loop and sinusoidal shortening contractions is illustrated in Figure 1). The work loops were performed at each of four cycle frequencies (0.5, 1, 1.5, and 2 Hz). Each work loop protocol was composed of two passive cycles, one active cycle, and a final passive cycle. This protocol allowed for the analysis of the second (passive) and third (active) cycle, which were not influenced by discontinuities associated with starting and stopping the servo-tube. Stimulation onset occurred at maximum length, and offset occurred prior to the end of shortening to allow for complete relaxation of the muscle immediately prior to subsequent lengthening. This stimulation scheme was developed to minimize negative work (i.e., eccentric contractions) while maximizing positive work (Caiozzo and Baldwin, 1997). The discrete shortening protocols consisted of a passive lengthening phase, two-second pause, active shortening phase, two-second pause, followed by a second passive lengthening phase that returned the muscle to the initial position. Within these protocols, the muscle length changes during the shortening phase followed a half-sinusoidal trajectory identical to the active shortening phase during the work loops. Stimulation during the discrete shortening contractions was similar to the work loops in that it started with the onset of the shortening phase; however, unlike the work loop condition, stimulation during the sinusoidal shortening contractions continued through the end of the shortening. Hence, the two protocols differ only in terms of stimulation offset, the difference of which represents the time required for complete muscle relaxation. This set of work loops (0.5, 1, 1.5, and 2 Hz) and matched sinusoidal shortening contractions were performed twice, once across muscle lengths that represented walking gait and once centered about Lo. To minimize fatigue effects, all protocols were separated by two minutes and performed in a randomized order. A 400 ms isometric contraction was performed after every fourth protocol to monitor fatigue. Extra recovery was given if fatigue was evident and the investigation was terminated if isometric forces did not recover.
Figure 1.
Position trajectory for work loop (1 Hz, 10 mm excursion) and matched discrete shortening protocols. Stimulation onset for both protocols is indicated by solid lightening bolt, while offsets are indicated with empty black and grey lightening bolts for work loops and discrete shortening protocols respectively.
For both protocols, active force during the shortening phase was determined by subtracting force during the passive cycle (passive forces) from force during the active cycle (net forces) at equivalent muscle lengths. Work performed during work loops was calculated by finite differentiation (first central difference method) of active force and shortening distance during the work loops. Likewise work performed during discrete shortening contractions was calculated by finite differentiation of active force and shortening distance. For each cycle frequency, work difference (ΔW) was calculated as the difference between work performed during work loops and work performed during discrete shortening contractions.
A two by two by four repeated measures ANOVA was used to determine the effects of stimulation offset (i.e., work loop or discrete contraction), muscle length (representing walking gait or centered on Lo) and cycle frequency (0.5, 1, 1.5 and 2 Hz) on the muscles’ abilities to perform work. In addition, a two by four repeated measures ANOVA was used to determine the influence of muscle length and cycle frequency on the difference in work production between work loop and discrete shortening protocols. The Huynh-Feldt correction was used when data violated the assumption of sphericity. Pairwise comparisons were used to further elucidate where significant simple effects existed if the main effects were found to be significant (alpha adjusted with LSD). All comparisons were performed at the α level of 0.05.
Results
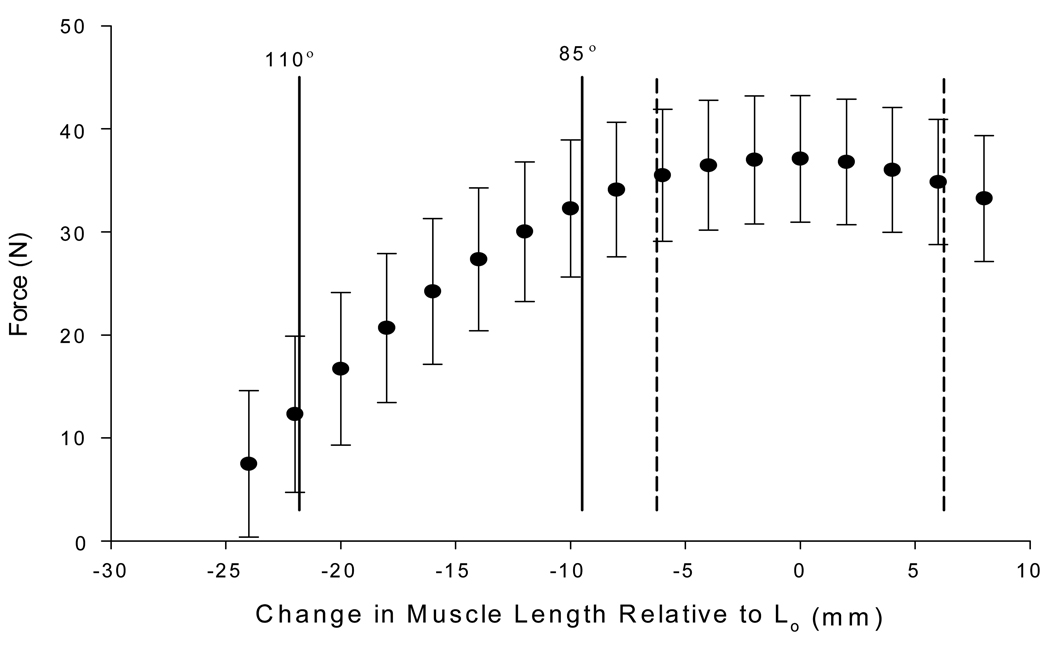
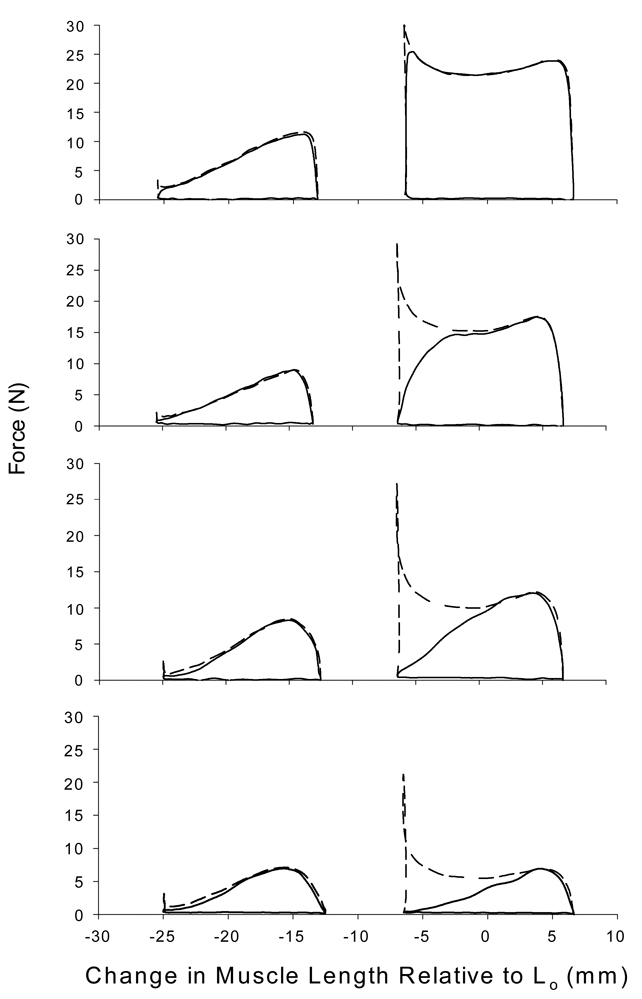
The methods utilized to determine muscle length excursions resulted in a pair of dynamic protocols with non-overlapping muscle length ranges (Figure 2). The muscle length excursions that represented walking gait (ankle angle range of 85–110°) remained exclusively on the ascending limb of the force-length relationship, whereas the muscle length excursions that centered on Lo remained near the plateau of the force-length relationship. The mean (± SD) muscle length change for the shortening phase of both protocols was 12.25 ± 2.0 mm. Representative force-length trajectories for both protocols and at both muscle lengths are illustrated in Figure 3.
Figure 2.
Average (±SD) force-length relationship for all four cats. Lo is represented by 0 on the x-axis. Solid vertical lines represent the average range of motion used during excursions that represented walking gait. Dashed vertical lines represent the average range of motion used during excursions centered on Lo.
Figure 3.
Representative force-length trajectories from a single cat. Force-length trajectories in the left column represent walking gait excursion and those in the right column center about Lo (indicated by 0). The dashed line depicts force traces during discrete shortening contractions whereas the solid line depicts force traces during work loops.
The main effects under investigation were stimulation offset (represented by work loop or discrete shortening contractions), muscle length (representing walking gait or centered on Lo) and cycle frequency (0.5, 1, 1.5, and 2 Hz). Repeated measures ANOVA indicated the main effects of stimulation offset, muscle length and cycle frequency were significant. The ANOVA also indicated that the following interactions were also significant: contraction type by muscle length and contraction type by cycle frequency. The muscle length by cycle frequency interaction (p = 0.051, power = 0.572) displayed a strong trend toward significance.
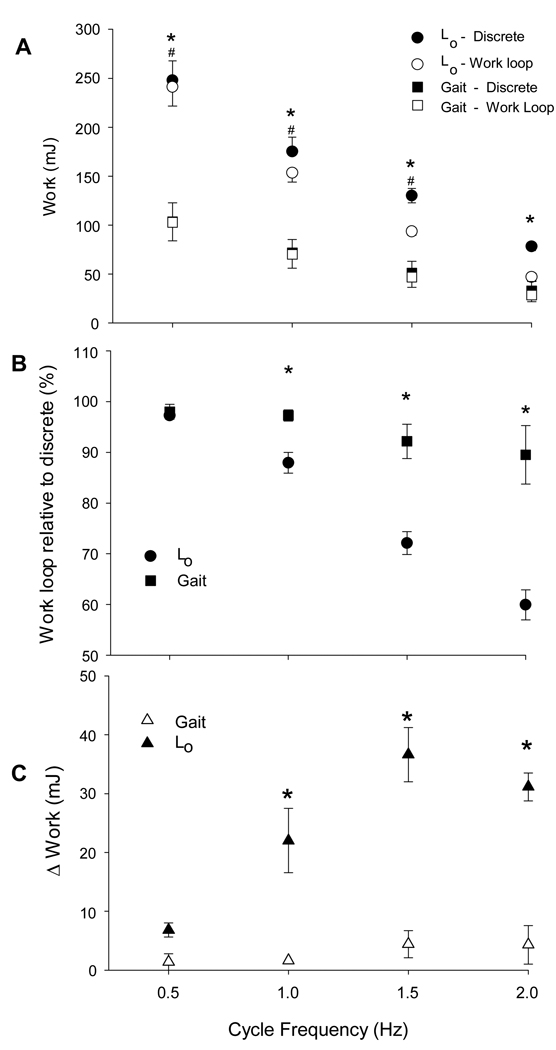
For muscle length excursions centered on Lo, pairwise comparison indicated a significant difference between the work performed during work loops and discrete shortening contractions for all cycle frequencies (Figure 4A). This difference (mean ± SD) increased with cycle frequency (2.8 ± 0.8%, 12.1 ± 4.1%, 27.9 ± 4.5% and 40.1 ± 5.9% for 0.5, 1, 1.5, and 2 Hz, respectively). For muscle length excursions representing walking gait, pairwise comparison did not indicate significant differences between work performed during work loops and discrete shortening contractions at any cycle frequency (Figure 4A). This interaction is also depicted when work performed during work loops is expressed as a percentage of work performed during discrete shortening contractions (Figure 4B). When muscle length excursions were centered on Lo, the work performed during work loops decreased by up to 41% compared with the discrete shortening contractions. A decrease in work not more than 10% was observed when the muscles operated over lengths that represented walking gait. Hence, the limitations of relaxation kinetics were four times greater when muscle length excursion centered on Lo compared to those representing walking gait.
Figure 4.
The effects of relaxation kinetics, cycle frequency and muscle length on muscular work. A: Average work performed during work loops and discrete shortening contractions centered across Lo and at lengths that represent walking gait. (* indicates significant differences between work produced during work loops and discrete shortening contractions across Lo. # indicates significant differences between work produced during work loops centered about Lo and those that represent gait excursions.) B: Work produced during work loops expressed as a percentage of work produced during discrete shortening contractions. C: The difference in work between work loops and discrete shortening contractions. For B and C, * indicates significant difference within each cycle frequency.
Repeated measures ANOVA indicated a significant effect for muscle length, cycle frequency and the muscle length by cycle frequency interaction on the difference in work between work loops and discrete shortening contractions. When muscle length excursions were centered on Lo, work difference increased to a max of 36.6 ± 9.3 mJ as cycle frequency increased to 1.5 Hz compared to only a 4.42 ± 4.0 mJ difference, also at 1.5 Hz, when excursions represented walking gait (Figure 4C). Pairwise comparison indicated work difference to be greater for muscle length excursions centered on Lo compared to those that represented walking gait for 1 Hz, 1.5 Hz and 2 Hz but not for 0.5 Hz (p = 0.084) cycle frequencies.
The ability for the muscle to perform work was dependent on the muscle lengths through which it shortened. Pairwise comparisons indicated that work performed during work loops was greater for muscle length excursions centered on Lo compared to excursions represented walking gait for 0.5 Hz, 1 Hz and 1.5 Hz but not for 2 Hz (p = 0.109) cycle frequencies (Figure 4A- empty symbols). The mean (± SD) absolute and relative differences in work between the two muscle lengths were 138.2 ± 65.6mJ (55.7 ± 20.0%), 83.1 ± 36.7mJ (53.5 ± 21.0%) and 46.7 ± 20.5mJ (50.1 ± 22.0%) for 0.5 Hz, 1 Hz and 1.5 Hz, respectively.
Discussion
In this investigation we hypothesized that relaxation kinetics would limit muscular work when muscle length excursions occurred across Lo and when they represented walking gait. The primary findings of this investigation demonstrated that limitations imposed by relaxation kinetics on work were dependent on both cycle frequency and muscle length. More specifically, the influence of relaxation kinetics increased as cycle frequency increased, and was much greater for excursions centered on Lo compared to those that represented walking gait. A secondary finding of this investigation demonstrated that the capacity of cat soleus muscle to perform work across walking gait excursions was significantly less than the capacity to perform work across Lo. These results imply that relaxation kinetics are important factors that limit cyclic muscular work; however, its influence on physiological function depends on the relation between the natural length excursion of the muscle and Lo.
Limitations of Relaxation Kinetics are Length Dependent
The limitations imposed by relaxation kinetics were dependent on muscle length. Large differences between work performed during work loops and discrete shortening contractions occurred for muscle length excursions centered on Lo, and no significant differences occurred for excursions representing walking gait (Figure 4), which occurred exclusively on the ascending limb of the force-length curve (Figure 2). Furthermore, while collecting pilot data (not reported), similar comparisons were made using muscle length excursions that centered on Lo, and excursions that were midway between Lo and lengths that represented walking gait. Those data suggested that relaxation kinetics limited work, but not to the extent they did when excursions were centered on Lo. Hence, it seems that a relationship exists between muscle length, with respect to the force-length curve, and the extent to which relaxation kinetics limits work.
The force-length trajectories (Figure 3) illustrate why relaxation kinetics impose such a minor role on the ascending limb of the force-length relationship. The figures in the right column represent a typical force-length trajectory during work loops (solid line) and discrete shortening contractions (dashed line) for muscle excursions centered on Lo. As the muscle shortens during a discrete shortening contraction, the force trajectory is largely dictated by force-velocity properties. The muscle is at its highest velocity half way through the shortening phase and slowest at the beginning and end of the shortening phase because of the sinusoidal profile. Hence, force is lowest midway through the shortening phase and highest at the end (activation kinetics limit force at the beginning of the shortening phase especially at faster cycle frequencies). During work loop conditions, stimulation must be discontinued prior to the end of the shortening phase to allow for complete relaxation of the muscle during subsequent lengthening. As a result, work is lost due to relaxation kinetics (area between the solid and dashed lines). In contrast, the excursions that represent walking gait (left column of Figure 3) occur on the ascending limb of the force-length relationship (Figure 2). At the end of these excursions the muscle is unable to produce much force due to force-length effects. Hence, when stimulation is stopped early during work loops, the amount of work that could potentially be lost is minimal.
In light of these findings it should be noted that stimulation pulse trains were longer during work loops representing walking gait compared to those centered on Lo. As mentioned previously, the pulse train durations were set independently for all eight combinations of cycle frequency and muscle length to minimize eccentric contractions yet maximize work. As a result stimulation duration was 1.4%, 15.1%, 40.8%, and 105.3% longer for 0.5 Hz, 1 Hz, 1.5 Hz, and 2 Hz work loops that represented walking gait compared to those that centered on Lo. The smaller forces and increased relaxation rate (Caiozzo and Baldwin, 1997, Rassier and MacIntosh, 2002, Brown and Loeb, 2000) at shorter muscle lengths, those representing walking gait, allowed for the longer stimulation pulse train.
For muscle length excursions centered on Lo, the absolute difference between work performed during work loops and discrete shortening contractions increased and their ratio decreased as cycle frequency increased from 0.5 to 2 Hz (Figure 4). These results support previous findings by Caiozzo and Baldwin (1997) who reported that the ratio of actual muscular work to theoretical work also decreased with increased cycle frequency. Factors not included in their model, such as force-length properties and shortening induced deactivation (Abbott and Aubert, 1952, Herzog et al., 2000, Josephson and Stokes, 1999, Meijer, 2002, Herzog et al., 1998), might further influence limitations imposed by relaxation kinetics. In place of their theoretical model we used discrete sinusoidal shortening contractions. The stimulation onsets and position trajectories of the active shortening phase during the sinusoidal contractions were matched to the stimulation onsets and position trajectories active shortening phase in the work loops (Figure 1). As a result, both conditions included history-dependent effects as well as force-length effects, and the differences in work between the two conditions can only be accounted for by relaxation kinetics.
Walking Gait Length Excursion is Located on Ascending Limb of Force-Length Relationship
In the current investigation we related ankle angles to displacement of soelus tendon markers relative to tibia markers to determine muscle lengths associated with walking gait. Results from these measurements placed the entire walking gait range on the ascending limb of the force-length relationship and Lo at approximately 40° (Figure 2). This is in agreement with data reported by Herzog, Leonard, Renuad et al. (1992) who reported max isometric force for cat soleus to be at 30–50° ankle angle. Based on kinematic data, they also reported that the muscle remains on the ascending limb of the force-length curve throughout the functional range of motion of 60–140°. This discrepancy between muscle lengths associated with Lo and physiological range of motion explains the large decrement in work production when muscle length excursions represent walking gait compared to being centered about Lo. However, the moment arm of the soleus about the ankle is maximal near 100° ankle angle (Young et al., 1992), which is located near the middle of the walking gait ankle range. Therefore when considering ankle torque and the ability to transmit force to the external environment, location of maximum moment arm at this ankle angle may partially offset decreasing muscular force due to the force-length effects.
It could be hypothesized that relaxation kinetics would impose even larger limitations to work when muscle length excursions are centered on the descending limb of the force-length curve. For example, the physiological range for frog semitendinosus muscle occupies primarily the descending limb of the isometric force-length relationship, and is thought to approach peak forces while shortening during jumping (Mai and Lieber, 1990). If cyclic contractions were imposed on this muscle across physiological muscle lengths, one could expect stimulation to be turned off very early to allow for complete relaxation prior to lengthening. In this situation, relaxation kinetics would severely limit the muscle’s ability to produce work, even more so than observed in the current investigation. However, relaxation kinetics may not necessarily limit work during frog’s functional locomotory activity considering frogs main form of locomotion does not require cyclic contractions, rather it includes a long flight phase between contractions. Thus two follow up questions stem from these results: 1) Does the alterations in force-length properties as a result of the chronic muscular requirements (27) minimize the limitations of relaxation kinetics, and 2) Does fatigue enhance the limitations of relaxation kinetics when muscle length excursions are on the ascending limb of the force-length relationship, or represent a physiological range of motion, as it does when muscle length excursions are centered on Lo (Syme and Tonks, 2004, Askew et al., 1997)?
Current Model and Voluntary Walking Gait
The muscle length excursions and stimulations patterns had similarities to those observed during voluntary walking. The ankle angle range (85–130°) and average muscle length excursion it produced (12.25 mm) are in agreement with previous reports that have described the ankle angles and muscle length excursions used during normal cat gait ((Goslow et al., 1973, Gregor et al., 2006, Carlson-Kuhta et al., 1998, Gregor et al., 1988)). However, in our model, stimulation was initiated at the beginning of the shortening phase (a strategy that minimizes negative work (Caiozzo and Baldwin, 1997), whereas in normal walking stimulation may be initiated while the muscle is still lengthening (Gregor et al., 1988, Gregor et al., 2006, Trank et al., 1996, Carlson-Kuhta et al., 1998). Furthermore, in the current protocol the shortening phase consisted of an uninterrupted sinusoidal length trajectory and did not include the eccentric phase associated with normal walking when the ankle extensors accept the weight of the cat during ground contact (Goslow et al., 1973, Trank et al., 1996, Carlson-Kuhta et al., 1998). Consequently, the muscles in the current study were not able to take advantage of neither augmented force during shortening due to preactivation nor the stored strain energy that is recovered during normal walking gait. It has been reported that during locomotion the activation of the soleus muscle is turned off far in advance of the lengthening phase, resulting in the complete relaxation of the soleus muscle immediately prior to the impending lengthening phase (Whiting et al., 1984, Gregor et al., 1988). This characteristic was similar to stimulation offset in the current investigation. In fact, our stimulation and position trajectory may be more representative of cat incline walking gait in which there is a decreased preactivation (Gregor et al., 2006) and decreased ankle flexion upon paw ground contact (Gregor et al., 2006, Carlson-Kuhta et al., 1998).
Conclusion
In this investigation, work lost due to relaxation kinetics was measured by comparing the muscles’ ability to perform work during discrete shortening contractions to cyclic contractions, in which stimulation was turned off prior to the end of shortening. These results indicated relaxation kinetics imposed major limitations to work performance during cyclic contractions. Furthermore, the extent to which relaxation kinetics limited work was dependent on cycle frequency and muscle length. A secondary finding of this investigation supported cat soleus muscle had a significantly reduced ability to perform work at lengths which represent walking gait compared to muscle length excursions centered on Lo. These results imply that relaxation kinetics are important factors that limit cyclic muscular work; however, its influence on physiological function may depend on the relation between natural excursion of the muscle and Lo.
Acknowledgments
Funding
National Institute of Health: RO1NSO39677
Footnotes
Conflict of Interest
None declared
REFERENCES
- Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. Journal of Physiology. 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- Askew GN, Marsh RL. Optimal shortening velocity (V/Vmax) of skeletal muscle during cyclical contractions: length-force effects and velocity-dependent activation and deactivation. Journal of Experimental Biology. 1998;201:1527–1540. doi: 10.1242/jeb.201.10.1527. [DOI] [PubMed] [Google Scholar]
- Askew GN, Young IS, Altringham JD. Fatigue of mouse soleus muscle, using the work loop technique. Journal of Experimental Biology. 1997;200:2907–2912. doi: 10.1242/jeb.200.22.2907. [DOI] [PubMed] [Google Scholar]
- Branner A, Stein RB, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. Journal of Neurophysiology. 2001;85:1585–1594. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- Brown IE, Loeb GE. Measured and modeled properties of mammalian skeletal muscle: IV. dynamics of activation and deactivation. Journal of Muscle Research and Cell Motility. 2000;21:33–47. doi: 10.1023/a:1005687416896. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Baldwin KM. Determinants of work produced by skeletal muscle: potential limitations of activation and relaxation. American Journal of Physiology. 1997;273:C1049–C1056. doi: 10.1152/ajpcell.1997.273.3.C1049. [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. Journal of Neurophysiology. 1998;79:1687–1701. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. Journal of Morphology. 1973;141:1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Roy RR, Whiting WC, Lovely RG, Hodgson JA, Edgerton VR. Mechanical output of the cat soleus during treadmill locomotion: in vivo vs in situ characteristics. Journal of Biomechanics. 1988;21:721–732. doi: 10.1016/0021-9290(88)90281-3. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. Journal of Neurophysiology. 2006;95:1397–1409. doi: 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- Herzog W, Guimaraes AC, Anton MG, Carter-Erdman KA. Moment-length relations of rectus femoris muscles of speed skaters/cyclists and runners. Medicine and Science in Sports and Exercise. 1991;23:1289–1296. [PubMed] [Google Scholar]
- Herzog W, Leonard TR, Wu JZ. Force depression following skeletal muscle shortening is long lasting. Journal of Biomechanics. 1998;31:1163–1168. doi: 10.1016/s0021-9290(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Herzog W, Leonard TR, Wu JZ. The relationship between force depression following shortening and mechanical work in skeletal muscle. Journal of Biomechanics. 2000;33:659–668. doi: 10.1016/s0021-9290(00)00008-7. [DOI] [PubMed] [Google Scholar]
- James RS, Young IS, Cox VM, Goldspink DF, Altringham JD. Isometric and isotonic muscle properties as determinants of work loop power output. Pflugers Archiv European Journal of Physiology. 1996;432:767–774. doi: 10.1007/s004240050197. [DOI] [PubMed] [Google Scholar]
- Josephson RK, Stokes DR. Work-dependent deactivation of a crustacean muscle. Journal of Experimental Biology. 1999;202:2551–2565. doi: 10.1242/jeb.202.18.2551. [DOI] [PubMed] [Google Scholar]
- Mai MT, Lieber RL. A model of semitendinosus muscle sarcomere length, knee and hip joint interaction in the frog hindlimb. Journal of Biomechanics. 1990;23:271–279. doi: 10.1016/0021-9290(90)90017-w. [DOI] [PubMed] [Google Scholar]
- Marsh RL. Deactivation rate and shortening velocity as determinants of contractile frequency. American Journal of Physiology. 1990;259:R223–R230. doi: 10.1152/ajpregu.1990.259.2.R223. [DOI] [PubMed] [Google Scholar]
- Meijer K. History dependence of force production in submaximal stimulated rat medial gastrocnemius muscle. Journal of Electromyography and Kinesiology. 2002;12:463–470. doi: 10.1016/s1050-6411(02)00040-8. [DOI] [PubMed] [Google Scholar]
- Rassier DE, MacIntosh BR. Length-dependent twitch contractile characteristics of skeletal muscle. Canadian Journal of Physiology and Pharmacology. 2002;80:993–1000. doi: 10.1139/y02-127. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Annals of Biomedical Engineering. 1992;20:413–422. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- Stevens ED. The pattern of stimulation influences the amount of oscillatory work done by frog muscle. Journal of Physiology. 1996;494:279–285. doi: 10.1113/jphysiol.1996.sp021490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme DA, Tonks DM. Fatigue and recovery of dynamic and steady-state performance in frog skeletal muscle. American Journal of Physiology. 2004;286:R916–R926. doi: 10.1152/ajpregu.00347.2003. [DOI] [PubMed] [Google Scholar]
- Trank TV, Chen C, Smith JL. Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. Journal of Neurophysiology. 1996;76:2316–2326. doi: 10.1152/jn.1996.76.4.2316. [DOI] [PubMed] [Google Scholar]
- Whiting WC, Gregor RJ, Roy RR, Edgerton VR. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. Journal of Biomechanics. 1984;17:685–694. doi: 10.1016/0021-9290(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Young RP, Scott SH, Loeb GE. An intrinsic mechanism to stabilize posture--joint-angle-dependent moment arms of the feline ankle muscles. Neuroscience Letters. 1992;145:137–140. doi: 10.1016/0304-3940(92)90005-r. [DOI] [PubMed] [Google Scholar]