Table 1.
Reduction of ketones containing the furanyl ring and using spiroborate ester 1 in 10 and 1 mol %a
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Cat. 1 (%) |
Yieldb (%) |
Eec (%) |
| 1 |  |
10 | 63 | 95 |
| 2 | 1 | 65 | 95 | |
| 3 |  |
10 | 87 d | 91 |
| 4 | 1 | 91 | 85 | |
| 5 |  |
10 | 88d | 96 |
| 6 | 1 | 95 | 96 | |
| 7 | 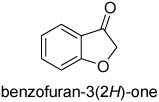 |
10 | 86d | 92 |
| 8 | 5 | 78d | 92 | |
| 9 |  |
10 | 82 | >99e |
The reaction was carried out using 0.7 equiv of borane DMS in THF at room temperature.
Yield after distillation in a Kugelrohr apparatus.
Determined by GC of the acetate on a CP-Chirasil-Dex CB column.
Yield after column chromatography.
Determined by 31P NMR of a phosphonate (CDA) derivative.15
