Table 2.
Reduction of heteroaryl ketones containing sulfur using 1and 10 mol % of spiroaminoborate ester 1.a
| Entry | Substrate | Cat. 1 (%) |
Yieldb (%) |
Eec (%) |
|---|---|---|---|---|
| 1 |  |
10 | 92 | 98 |
| 2 | 1 | 87 | 96 | |
| 3 | 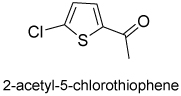 |
10 | 91(83)d | 98 (99)d |
| 4 | 1 | 91 | 98 | |
| 5 |  |
10 | 75 | 97 |
| 6 | 1 | 86 (71)d | 98 (82)d | |
| 7 |  |
10 | 90 (83)d | 95 (99)d |
| 8 | 1 | 89 | 96 | |
| 9 |  |
10 | 92 (76)d | 99(99)d |
| 10 | 1 | 92 | 99 | |
| 11 |  |
10 | 92(53)d | 94.5(94)d |
| 12 | 1 | 93 | 94 | |
| 13 |  |
10 | 80c,d | 93d |
| 14 | 1 | 92 | 99 (92)d | |
| 15 |  |
10 | 85c | 100 |
| 16 | 1 | 92 | 99 | |
The reaction was carried out using 0.7 equiv of borane DMS in THF at room temperature.
Isolated yield after distillation in a Kugelrohr apparatus.
Determined by GC of the acetate on chiral column.
The reaction was carried out using BH3-DEA
