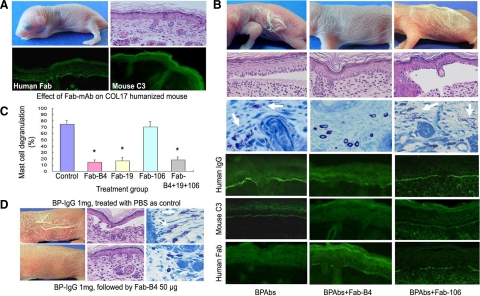
Figure 7.
Therapeutic effects of Fabs on BP model mice. A: Results of the injection of Fabs into neonatal COL17 humanized mice show that this treatment alone does not cause BP disease or other detectable adverse effects. Histological examination (right, upper panel) supports this result. Indirect immunofluorescence show DEJ staining for the recombinant Fabs (left of lower panel) but no staining for mouse C3 (right, lower panel). B: Mice injected with NC16A affinity purified BPAbs develop the clinical and histological skin detachment associated with MC degranulation (white arrows) and the deposition of human IgG and mouse C3 at the DEJ. In contrast, mice injected with BPAbs and Fab-B4 fail to show these clinical and histological characteristics, and the intensity of IgG deposition at the DEJ is markedly reduced. The staining of mouse C3 is absent, whereas recombinant Fab fragment staining is weak but detectable. Fab-106 fails to show any beneficial therapeutic effect in the animal model. C: Percentage of dermal MC degranulation is assessed in BP model mice and in those treated with 30 μg/g body weight of Fabs. It is significantly reduced in the mice treated with Fab-B4, Fab-19, and the Fab combination. *P < 0.01 versus control group (BP model mice treated with PBS). D: BP model mice were produced by injection of BP-IgG (total IgG fraction prepared from BP patients) and were treated with Fab-B4 24 hours later. BP-like clinical and histological characteristics fail to develop in most (four of five) of Fab-B4 treated mice (lower panel).