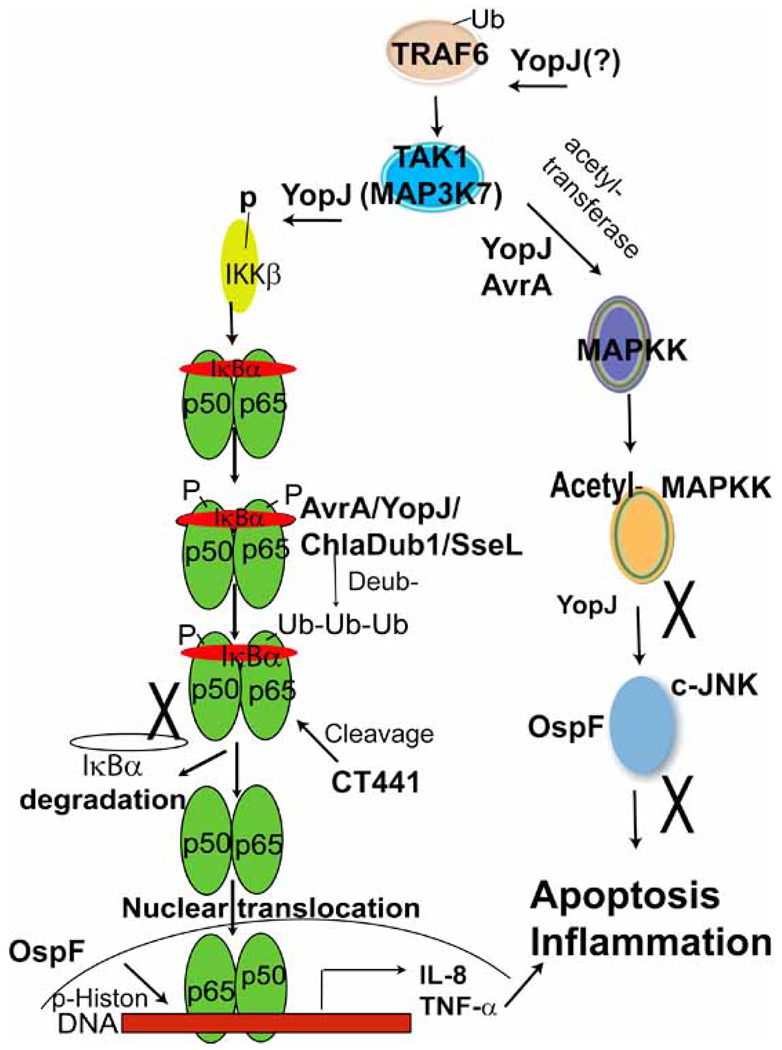
Fig. (2). Inactivation of NF-κB and MAPK pathways (simplified version) by the bacterial proteins.
As shown here, MAPK signaling through sequential steps involves TRAF6, TAK1, and MAPKK. A parallel NF-κB pathway involves TRAF6, TAK1, IKKβ, IκBα phosphorylation (P), ubiquitination (Ub), NF-κB translocation, activation of NF-κB transcription, and target gene expression. Bacterial TTSS effectors including Salmonella AvrA, SseL, Yersinia YopJ, and Chlamydia ChlaDub1/2 act as deubiquitinases to target the ubiquitination of IκBα, thus inhibiting the NF-κB activity. Chlamydia protein CT441 cleaves the NF-κB subunit p65 and attenuate the NF-κB activity. Additionally, Shigela OspF modifies phosphorylation of histone H3 at Ser10 increases chromatin accessibility to transcription factor NF-κB. Acting as a broad-specificity deubiquitinase, YopJ removes Ub from proteins, including TRAF6 and IκBα. MAPKK and IKKβ are activated by phosphorylation of serine or threonine residues in their activation loops. YopJ inactivates these pathways. The MAPK pathway is modulated by AvrA through acetylation. AvrA, YopJ, and OspF inhibit the c-JNK pathway, attenuate the inflammatory responses, and interfere with the cell apoptotic process.