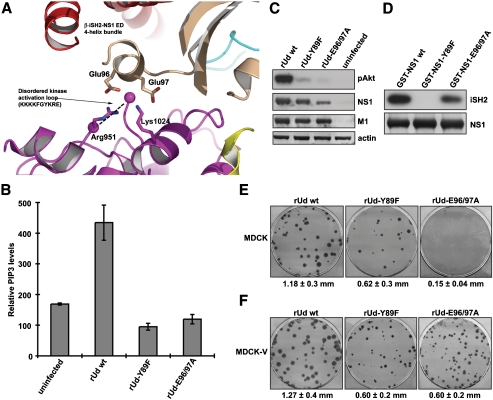
Fig. 4.
Roles of the NS1 acidic α-helix in both PI3K activation and IFN-antagonism. (A) Potential interaction between the NS1 ED and the p110α kinase activation loop. Domains of p85β, p110α, and NS1 ED are shown in cartoon representation. The edges of the disordered p110α activation loop are highlighted by spheres colored magenta. Specific residues are shown in stick representation. (B) The rUd-E96/97A virus does not activate PI3K. 1321N1 cells (which lack the PIP3 phosphatase, PTEN) (37) were infected with rUd WT, rUd-Y89F, or rUd-E96/97A viruses at an MOI of 5 PFU/cell for 10 h prior to analysis of total PIP3 levels. Bars show mean values obtained from triplicate samples assayed in duplicate. Error bars represent standard deviation. (C) A549 cells were infected as for (B). Cells were lysed and proteins analyzed by immunoblotting. The level of PI3K activation was determined by detection of phospho-Akt (Ser473). Viral NS1 and M1 proteins were detected as markers of infection. β-actin was used as a loading control. (D) GST-pulldown experiment showing the interaction of NS1 with β-iSH2. GST-NS1 proteins were immobilized onto agarose beads and incubated with 293T cell lysates expressing myc-tagged β-iSH2. NS1:β-iSH2 complexes were separated by SDS-PAGE and detected by immunoblotting using anti-NS1 and anti-myc antibodies. (E) Mean plaque size of recombinant rUd WT, rUd-Y89F, and rUd-E96/97A viruses in MDCK cells. Plaques were immunostained 3 days postinfection, and mean plaque size was determined (± SD). (F) Mean plaque size of recombinant rUd WT, rUd-Y89F, and rUd-E96/97A viruses in MDCK-V cells (targeted degradation of STAT1) (24). Plaques were immunostained 3 days postinfection, and mean plaque size was determined (± SD).