Figure 3.
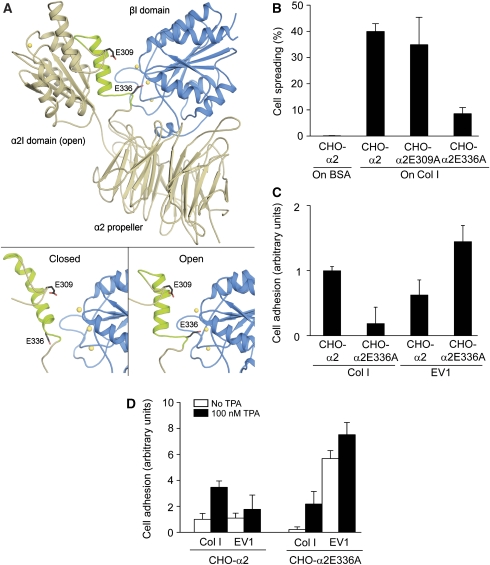
EV1 favours inactive α2β1 integrin. (A) A structural model of the human α2β1 integrin head region was built based on the crystal structures of the αVβ3 integrin and the α2I domain. (A; bottom, left) Modelling indicates that the closed conformation of the α2I domain does not form specific contacts with the β1I domain (blue). (A; bottom, right) However, when the α2I domain adopts an open conformation, E336 at the C-terminal end of helix α7 is positioned in such a way that it could coordinate to the metal ion (yellow) of the MIDAS in the β1I domain and act as an intrinsic ligand. E309 does not seem to participate in the process. (B) In a cell spreading assay, CHO-α2 and CHO-α2E309A cells attached and spread on a collagen I (Col I) matrix in 120 min, whereas α2E336A mutation caused a dramatic decrease in the spreading. Cells were not able to attach to or spread on BSA, which was used as a negative control. (C) When CHO-α2 and CHO-α2E336A cells were allowed to adhere to immobilized EV1 or collagen I for 15 min, the E336A mutation seemed to decrease α2-mediated cell adhesion to collagen I. Interestingly, EV1 seemed to favour CHO-α2E336A cells. Mean values±s.d. of four parallel measurements are shown (B, C). (D) However, at 15 min, CHO-α2 and CHO-α2E336A cell adhesion to both collagen I and EV1 was significantly increased in the presence of integrin cluster-inducing TPA (100 nM; black columns). Mean values±s.d. of four parallel measurements are shown.