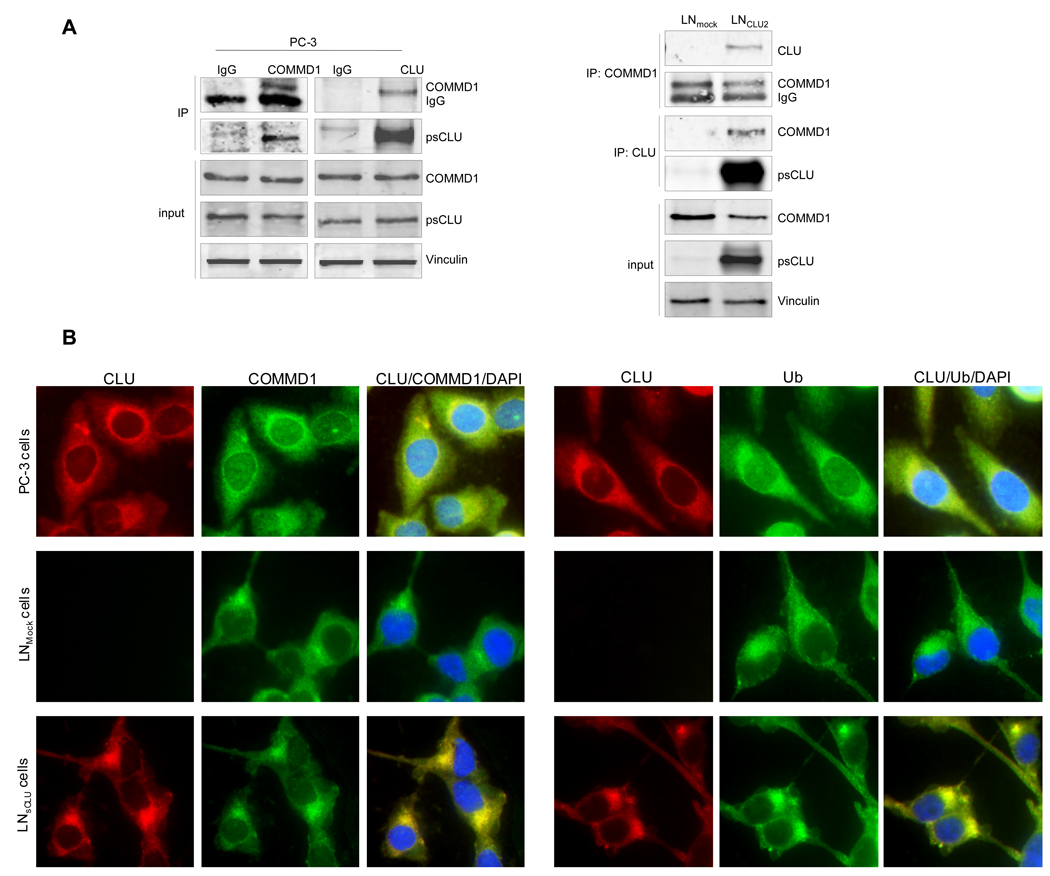
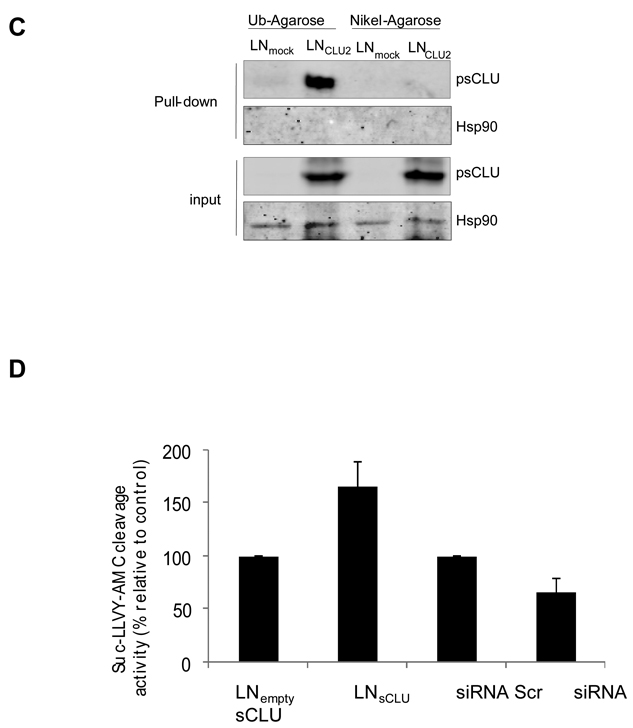
Figure 1. sCLU is a COMMD1 and ubiquitin partner in prostate cancer cells.
A, sCLU interacts with COMMD1. Total proteins from PC-3, LNmock and LNsCLU were co-IP with anti-CLU or normal IgG followed by western blot using COMMD1 antibody. The inverse experiment was performed using COMMD1 antibody for co-IP. CLU, COMMD1 and vinculin were used for western blot (sCLU is detected as a 60-kDa glycosylated presecretory form of sCLU and is referred as psCLU in all western blot as proposed by Trougakos (43)). B, sCLU co-localizes with COMMD1 and ubiquitin. Immunofluorescence was performed in PC-3, LNmock and LNsCLU using CLU, COMMD1, and ubiquitin antibodies and DAPI for nuclei staining. C, sCLU associates with ubiquitin. Total proteins were incubated with ubiquitin-agarose matrix or Nickel agarose; and bound protein and input were used for western blots with CLU and Hsp90 antibodies. D, sCLU enhances ubiquitinated proteins. Total proteins (50ug) from LNmock and LNsCLU or PC-3 treated with sCLU or Scr siRNA were monitored for cleavage of the Suc-LLVY-AMC. Fluorescence was quantified using a spectrofluorometer (Fluoroskan Ascent FL, Thermo Labsystem). Data is expressed as mean ± SE. *, statistical significance (p<0.05) from 3 biological replicates.