Figure 3. Analysis of peptide phosphorylation by Western blot and MALDI-TOF MS.
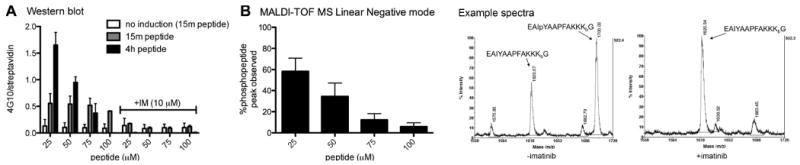
The engineered HEK293 cell line stably expressing a Tet-ON FKBP-Abl construct was plated at 2.5 × 106 cells per well in a six-well plate. Cells were allowed to grow to confluency (48 h) and treated with 2 μM doxycycline for 18h to induce the expression of FKBP-Abl. Abl-activating dimerizer AP20187 (10 nM) and peptide (at the indicated concentrations) were added and cells were incubated at 37 °C for 15 min and 4 hrs (with and without imatinib, 10 μM). A: Western blot analysis: Cell lysates were run on SDS-PAGE (200 μg/lane, 4-12% Bis-Tris gel) and biotinylated peptide (streptavidin-DyLight649) and phosphotyrosine (antiphosphotyrosine antibody 4G10) were detected with immunoblotting. Data are shown as phosphotyrosine signal divided by total peptide (4G10/streptavidin). B: MALDI-TOF analysis: Cell lysates (100 μg) were treated with UV light (302 nm) for 5 min. Peptide was isolated from the lysate via affinity capture with Tetralink Avidin beads using a 96-well filter plate. Peptides were eluted using 50/50/0.1% acetonitrile/H2O/TFA and analyzed using MALDI-TOF MS in linear negative mode. Example spectra: doxycycline-induced and AP20187-treated cells, incubation with 25 μM peptide for 4 h, +/- imatinib (10μM). See supporting information for representative blots and more MALDI spectra for these data.
