Figure 1.
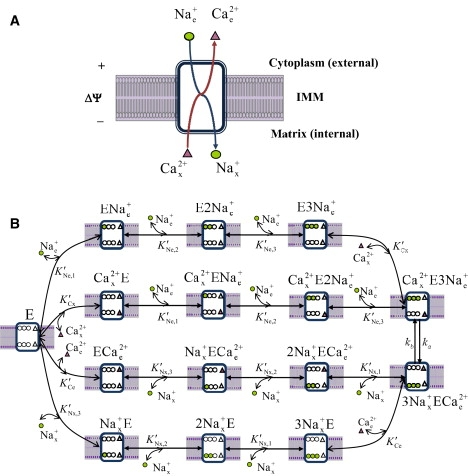
(A, B) Proposed kinetic mechanism of Na+-dependent Ca2+ efflux from mitochondria via Na+-Ca2+ antiporter with a presumed 3Na+:1Ca2+ stoichiometry. The antiporter E has three binding sites for Na+ and one binding site for Ca2+ facing either side of the IMM. In one process, three Na+ ions from cytoplasmic side first cooperatively bind to the unbound antiporter E in three consecutive steps to form the complex E3Nae+. Then one Ca2+ ion from the matrix side binds to the complex E3Nae+ to form the complex Cax2+E3Nae+. In another process, one Ca2+ ion from the matrix side first binds to the unbound antiporter E to form the complex Cax2+E. Then three Na+ ions from the cytoplasmic side cooperatively bind to the complex Cax2+E in three consecutive steps to form the complex Cax2+E3Nae+. The complex Cax2+E3Nae+ then undergoes conformational changes to form the complex 3Nax+ECae2+. The complex 3Nax+ECae2+ then undergoes the reverse processes, where it dissociates in two distinct processes to form three Na+ ions in the matrix side and one Ca2+ ion in the cytoplasmic side, in addition to the unbound antiporter E. K′Ne, p, K′Nx, p, K′Ce, and K′Cx are the apparent dissociation constants associated with the binding of external and internal Na+ and Ca2+ to the antiporter. The 3Na+:1Ca2+ exchange via the interconversion mechanism Cax2+E3Nae+ ↔ 3Nax+ECae2+ is limited by the forward and reverse rate constants ka and kb, which depend on ΔΨ.
