Figure 6.
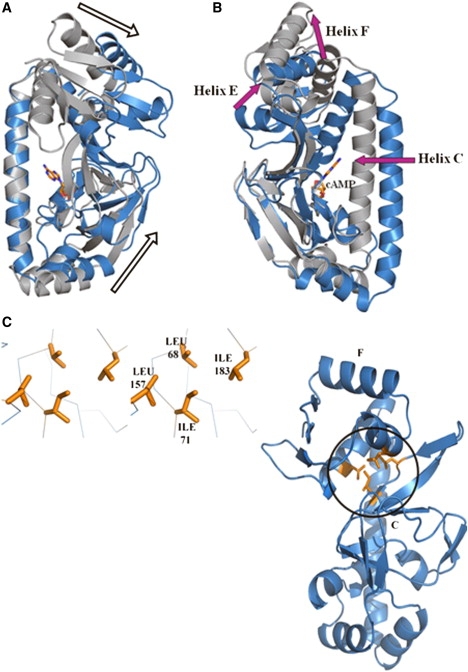
Change in the relative orientation between the cAMP-binding domain and the DNA-binding domain in CRPMt. (A) A stereo view of the superposition of apo (blue) and holo (gray) structures, when the C-helices are aligned, clearly shows that the cAMP- and DNA-binding domains move away from each other in the presence of cAMP. (B) An alternate view of conformational changes between the apo (blue) and holo (gray) structures is seen when the cAMP-binding domains are aligned. In this view, the C-helix is clearly shown to move toward the cAMP-binding domain in the presence of cAMP. (C) Due to the reorientation between the cAMP- and DNA-binding domains, a small hydrophobic core is formed at their interface in the apo structure. Two side chains each from the two domains contribute to the formation of the hydrophobic core. The two domains also now bury a larger accessible surface between them, as shown in Table 2.
