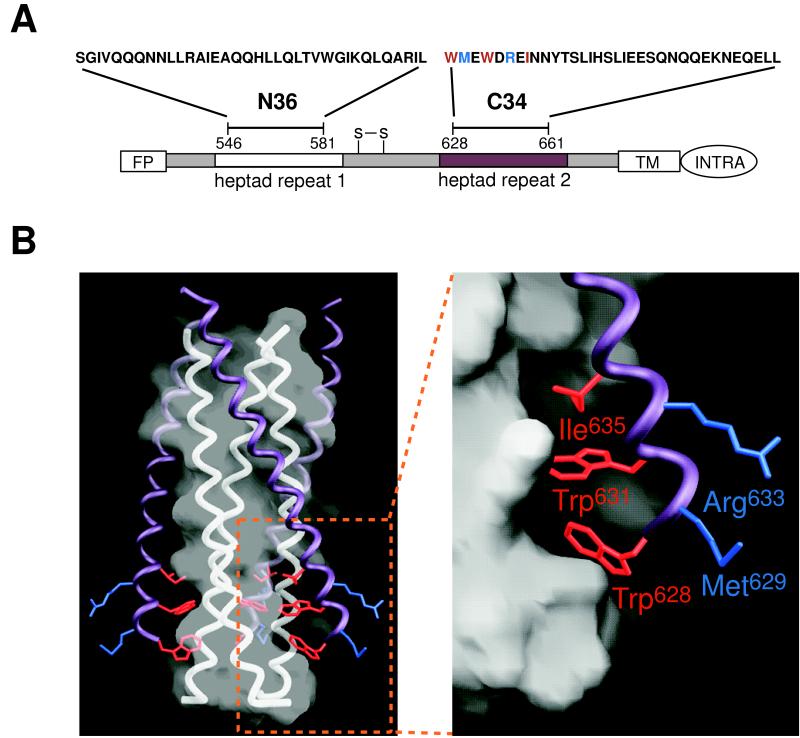
Figure 1.
HIV-1 gp41 structure and mutant peptides. (A) Schematic of HIV-1 gp41 showing the N36 and C34 peptides, located within two regions containing 4,3 hydrophobic heptad repeats (white and purple boxes). The residues corresponding to the peptides discussed in this paper are as follows: C34, 628–661; DP178, 638–673; T649, 628–663. All of these peptides are acetylated at the amino terminus and amidated at the carboxy terminus. Colored residues in C34 were mutated in this study. Red residues project into the N36 cavity, whereas blue residues do not. FP, fusion peptide; S-S, disulfide bond; TM, transmembrane region; INTRA, intraviral region. (B) The N36/C34 crystal structure of the HIV-1 gp41 ectodomain core (2). (Left) The trimeric N36 coiled coil is represented by white three helices overlaid with a semitransparent white molecular surface. Three C34 helices (shown in purple with selected side chains) pack against this coiled coil surface. The bottom of the N36 surface contains three symmetry-related cavities (one is outlined by the box), each of which accommodates three hydrophobic residues (red) from a C34 helix. In contrast, the blue residues project outward and do not make contacts with the coiled coil. (Right) A close-up of the cavity region with the C34 residues labeled. Here, the surface of the central coiled coil is represented by an opaque molecular surface. This figure was made with the program grasp (42).