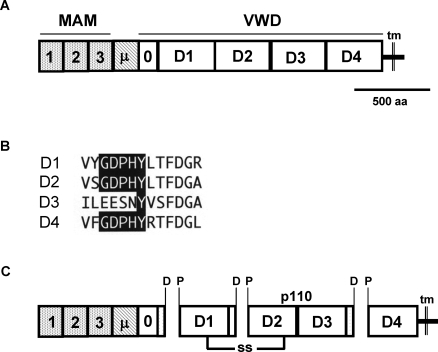
FIG. 6.
Domain structure and predicted processing of the horse zonadhesin precursor. The precursor's structural characteristics were inferred from its amino acid sequence deduced by conceptual translation of a composite cDNA. A) Predicted domain structure of the horse zonadhesin precursor. MAM, meprin/A5/receptor tyrosine phosphatase mu domain; μ, mucin domain; VWD, von Willebrand D domains; tm, transmembrane segment. B) Paralogous sequences in the horse zonadhesin precursor's VWD1, VWD2, VWD3, and VWD4 domains corresponding to sites of proteolytic processing in the pig protein. Note the conservation of the GDPHY sequence motif (shaded) in D1, D2, and D4. C) Predicted polypeptides produced by proteolytic cleavage of Asp-Pro bonds of GDPHY motifs during the functional maturation of horse zonadhesin. Note that a p110 polypeptide spans the VWD3 domain.