Abstract
Objectives
The influenza virus (IFV) infection models commonly used to evaluate antiviral agents (e.g. MDCK cell line and mice) are limited by physiological differences from the human respiratory tract in vivo. Here we report the pharmacodynamics of DAS181, a sialidase fusion protein that inhibits influenza infection, in the model systems of well-defined human airway epithelium (HAE) culture and ex vivo culture of fresh human bronchial tissue, both of which are close mimics of the human respiratory tract in vivo.
Methods
HAE culture and ex vivo human bronchi were used to evaluate the sialic acid removal and regeneration efficiency and IFV inhibition after various DAS181 treatment levels and regimens.
Results
DAS181 effectively desialylates HAE cultures and ex vivo bronchi tissues and therefore potently inhibits replication of different IFV strains. The treatment effect of DAS181 occurs immediately upon application to the epithelial surface and is unaffected by the respiratory mucus. In both HAE and human bronchial tissue, the inhibitory effect of DAS181 treatment lasts for at least 2 days. Approximately 80% epithelial surface desialylation and significant anti-IFV efficacy can be achieved at topical concentrations of DAS181 in the range of 5–10 µg/cm2 when applied once daily. An additional treatment or a higher loading dose of DAS181 on the first day provides significant additional treatment benefit. Comparing the effect of DAS181 versus its two analogues, DAS180 and DAS185, has confirmed that sialidase function is critical for DAS181, and the cell-binding domain (amphiregulin tag) prolongs DAS181 retention and potentiates its function.
Conclusions
These results provide valuable insights into DAS181 treatment dose and potential regimens in the clinical setting.
Keywords: HAE, ex vivo bronchi, Fludase
Introduction
Seasonal influenza virus (IFV) infection across the globe accounts for >250 000 deaths annually, and a pandemic influenza event may be of far greater magnitude. IFV vaccines are the most commonly used preventive agent against seasonal infection, but the high variability of IFVs from season to season dictates that vaccines need to be updated annually. Antiviral drug treatments directly targeting viral proteins have also been approved for use against IFV infection. However, resistance to most of the currently approved anti-influenza drugs [M2 blockers and the neuraminidase inhibitor (NAI) oseltamivir] has become common in seasonal influenza, highlighting a need for novel anti-influenza approaches.1,2 Targeting the host cell components required for viral infection is a novel antiviral approach that may have a reduced potential for generating drug resistance.
Sialic acids on the host cell surface are the primary receptors required for infection by all known IFV strains.3 The interaction between IFV haemagglutinin (HA) and sialic acid receptors on the host cell surface has long been known to be critical for IFV infection, although targeting this interaction as an anti-IFV therapeutic approach has never been realized until the generation of DAS181 (Fludase®), a recombinant fusion protein containing a sialidase catalytic domain and a respiratory epithelium-anchoring domain [amphiregulin (AR) tag].4 The sialidase activity of DAS181 can cleave α(2,6)- and α(2,3)-linked sialic acid receptors, which are preferentially recognized by human and avian IFV strains, respectively. DAS181 potently inhibits infection by seasonal IFV as well as the potentially pandemic H5N1 IFV strain in MDCK cells, mice and ferrets.4,5
DAS181 has been designed to function topically on the surface of the airway, and the drug product will be delivered as an oral inhalant. Thus, understanding the pharmacodynamics of DAS181 on the human airway epithelial surface is critical for selecting a drug treatment dose and regimen for clinical studies. Although the anti-IFV activity of DAS181 has been demonstrated in studies using cell lines and animal models, these model systems are dissimilar to the in vivo environment of human airway in some important aspects, which include lack of differentiated cells and mucus-secreting function in the cell line monolayers and, importantly, distinct composition and dynamics of sialylated glycans in animals and cell lines. Thus while being conventional model systems for anti-IFV activity study, mice and cell lines are not entirely appropriate for DAS181 pharmacodynamic analysis.
In this report, we evaluated DAS181 pharmacodynamics and anti-IFV activity in two model systems that closely mimic the human respiratory epithelium: well differentiated human airway epithelium (HAE) culture and ex vivo human bronchial tissue sections. Our results corroborate the previous findings on potent anti-IFV activities of DAS181, and provide valuable insights on treatment dose and regimen selection for DAS181, which is being tested in human clinical trials.
Materials and methods
Cells, tissues and viruses
Well-differentiated HAE cultures (wdHAE/HAE/Epi-airway, AIR196-HTS or Air-100) were purchased from Mattek Inc. (Ashland, MA, USA; www.mattek.com) and were maintained according to the supplier's instructions. The laboratory IFV strains A/Weiss/43 (H1N1), A/PR8/34 (H1N1), A/PortChalmers/1/73 (H3N2), A/Victoria/3/75 (H3N2) and B/Maryland/1/59 were obtained from the ATCC (Manassas, VA, USA). The low passage IFV clinical isolate A/HongKong/54/98 was isolated/cultured as described previously,6 while the low passage IFV clinical isolate A/HongKong/2637/04 was generously provided by Alexander Klimov (CDC). All virus samples were first minimally amplified on MDCK cells, and then aliquotted and stored at −80°C before use. HAE infection was carried out by incubation with viruses (5000–40 000 pfu/well diluted in PBS) on the apical side of the HAE for 1–2 h at 37°C followed by a single wash with PBS. Unless mentioned otherwise, all anti-IFV studies employed IFV A/PortChalmers/1/73 (10 000 pfu/well) as model virus.
For ex vivo culture, fresh bronchi tissue was removed from patients undergoing lung resection and immediately sectioned into multiple biopsies of ∼3 × 5 × 5 mm dimensions. After sectioning, the tissues were immediately placed into culture medium (F-12K nutrient mixture with l-glutamine and antibiotics) before infection and/or DAS181 treatment within 8 h of resection. Bronchi were infected at a titre of 1 × 106 TCID50/mL with A/HongKong/54/98 (H1N1), as described previously.7
Detection of the cell-bound DAS by a cell-based ELISA
HAE tissues were pre-treated with 100 µL of DAS181 or DAS180 at various concentrations for 2 h, rinsed twice with 200 µL of PBS, fixed with 0.05% glutaraldehyde for 10–15 min in PBS, and blocked with 3% BSA in PBS overnight at 4°C. The cell-bound DAS was detected with either a rabbit polyclonal antibody (pAb A106; 3 µg/mL) or a sheep pAb (A318; 1 µg/mL) raised against DAS181 for primary detection, and appropriate secondary pAb conjugated to horseradish peroxidase (HRP; 0.08 µg/mL) (similar results were obtained using a polyclonal peptide antibody that does not recognize the AR tag; data not shown). The cultures were washed and developed with the colorimetric substrate tetramethylbenzidine (TMB; Sigma, St Louis, MO, USA), stopped with 1 M H2SO4 and quantified at 450 nm. Wells incubated with secondary pAb alone represented the background control. The mean levels of cell-bound DAS181 in the samples that were fixed immediately after the 2 h study drug treatment was considered as 100% at t = 0. The percentage of DAS that remained cell bound during the subsequent observation period was calculated using the formula: [(average sample absorbance–average background absorbance)/(average DAS181 absorbance at t = 0–average background absorbance)] × 100.
Quantification of cell surface sialic acid level by enzyme-linked lectin assay (ELLA)
HAE cultures were treated, washed and fixed as for the DAS binding assay. Unless mentioned otherwise all experiments involved a 2 h exposure to DAS181; however, in some experiments various lengths of exposure to DAS181 (other than 2 h) were tested (Figure 1b). In all experiments DAS181 or virus was immediately applied to the HAE surface; however, in some experiments the cultures were pre-washed with PBS (five times with 200 µL) to remove mucus before treatment with DAS181 (Figure 1c). The cell surface sialic acid levels were quantified by an ELLA as previously described.4 HAE cultures were treated with DAS, washed, fixed, and blocked as above. Relative α2,6-linked sialic acid was detected with biotinylated SNA (Sambucus nigra) lectin (2 µg/mL; Vector Laboratories, Burlingame, CA, USA) for 2 h at 37°C. SNA is specific for Neu5Ac α(2,6)-Gal (α2,6-linked sialic acid). After the lectin incubation, the cells were washed four times in PBS + 0.1% Tween 20 (PBST). Secondary detection of the bound lectin was accomplished by incubating the cells with streptavidin–HRP (5 µg/mL; Vector Laboratories) for 1 h at 37°C. The signal was detected with TMB substrate as above. All samples were normalized such that 100% sialic acid was defined as the absorbance at 450 nm of untreated tissues and 0% sialic acid was defined as the absorbance at 450 nm of untreated tissues, without the lectin incubation step. The percentage of sialic acid remaining was calculated using 100% × [(absorbance of DAS181-treated cells – background)/(absorbance of untreated cells – background)]. Time course studies involved daily washing of the apical surface with PBS to approximately simulate normal mucociliary clearance.
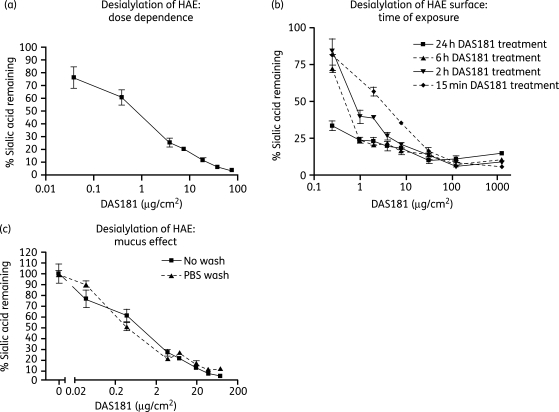
Figure 1.
DAS181 effectively removes sialic acid from the surface of HAE culture. To determine the dose–response, DAS181 was incubated with HAE cultures for 2 h at 37°C followed by immediate detection of surface levels of α2,6-sialic acid with lectin SNA (a). To determine the desialylation rate, DAS181 was incubated with HAE cultures for 0.25, 2, 6 or 24 h, before washing, fixation and immediate detection of surface levels of α2,6-sialic acid as above (b). To determine the effect of mucin, HAE cultures were either treated with DAS181 (2 h) and then fixed (No wash) or washed twice with 200 µL of PBS to remove mucins before treating with DAS181 and fixing (PBS wash) (c). Error bars represent the standard error of the mean of triplicate samples.
Time course analysis of DAS binding and HAE resialylation
HAE cultures were treated with DAS180 or DAS181 at 7.7 µg/cm2 for 2 h at 37°C followed by washing three times with 200 µL of PBS. Cultures were then incubated at 37°C for 10 days with daily PBS washing to mimic mucociliary clearance. At selected timepoints a subset of the cultures were fixed with 0.05% glutaraldehyde and stored in blocking buffer (3% BSA in PBS) at 4°C until analysis. DAS ELISA and SNA ELLA were performed as described above.
qRT–PCR methods
All studies involved daily washing of the apical surface with PBS, beginning 24 h post-infection, to simulate normal mucociliary clearance. The apical washes were collected and stored at −80°C until viral RNA was purified from 50 µL of wash sample using a MagMAX-96™ Viral RNA isolation kit (Applied Biosystems, Foster City, CA, USA). The RNA was purified according to the manufacturer's instructions and eluted in 50 µL of elution buffer. cDNA was synthesized with 10 µL of the purified viral RNA using an Applied Biosystems cDNA Synthesis kit. For the standard curve, 10-fold serial dilutions of the A/Weiss/43 M-gene were performed starting from 101 to 109 copies per quantitative reaction. A no-template control was included to control for cross-contamination. The quantitative PCR was carried out with 4 µL of cDNA, standard curve DNA or H2O for the no-template control. The 2X Fast Universal Master Mix (Applied Biosystems), 900 nM forward and reverse primers8 and 225 nM Taqman probe were added to the templates. PCRs were run under the conditions of one cycle of 95°C for 20 s, and 45 cycles of 95°C for 3 s and 60°C for 30 s on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems). Data were collected during the 30 s at 60°C. The Ct threshold was determined automatically and the Ct values were then plotted to form a standard curve by the Applied Biosystems Software. Typically, amplification with good linearity can be observed in 109 copies down to 102 copies. Copy numbers from the samples were interpolated with a fresh standard curve in each run.
Apoptosis array
HAE cultures (Air-100) were pre-treated with PBS or DAS181 (7.5 µg/cm2) for 2 h followed by washing with PBS and infection with 10 000 pfu of IFV B/Maryland/1/59. After incubation for 24 h at 37°C the cultures were washed with PBS and the culture/membrane was cut out of the plastic insert and submerged in RNAlater (Qiagen, Valencia, CA, USA).
Total RNA was extracted from ex vivo tissues using the RNeasy mini Kit (Qiagen). A 20 µL aliquot of the eluted total RNA was used for the first-strand cDNA synthesis with the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). The gene expression of 84 key apoptosis genes was then profiled by RT–PCR-based RT2 Profiler Apoptosis PCR Arrays (SABioscience, Frederick, MD, USA). RT–PCRs were performed in 96-well plate format using the ABI 7500 Real-Time PCR System (Applied Biosystems). Fold changes of apoptotic gene expression in experimental samples relative to the control samples (e.g. mock-infected) were calculated using the ΔCt method.9 The ΔCt value of each sample was normalized by up to a total of five housekeeping genes [β-2-microglobulin (B2M), hypoxanthine phosphoribosyltransferase 1 (HPRT1), ribosomal protein L13a (RPL13A), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB)]. All data were analysed by the RT2 Profiler PCR Array Data Analysis Template v3.0, and a 2.0-fold change in gene expression was used as the cut-off threshold to determine up- or downregulation, as previously described.10
IFV dynamics in HAE
In IFV time course studies ±DAS181 (Figure 4a), tissues were pre-treated with PBS or DAS181 for 2 h (153 µg/cm2) before infection with IFV A/PR8/34 (5000 pfu/well) for 1 h. After washing, the cultures were incubated at 37°C. Daily apical washes (100 µL/well) were collected and analysed for viral titre by plaque assay. In IFV time course studies comparing plaque assay and RT–PCR endpoints [Figure S1, available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/)] the cultures were simply infected with IFV A/PortChalmers/1/73 for 1 h (5000 pfu/well for the plaque assay experiment or 10 000 pfu/well for the RT–PCR experiment). After washing, the cultures were incubated at 37°C with daily apical washing (100 µL/well). Apical washes were collected and analysed for viral titre by plaque assay or RT–PCR.
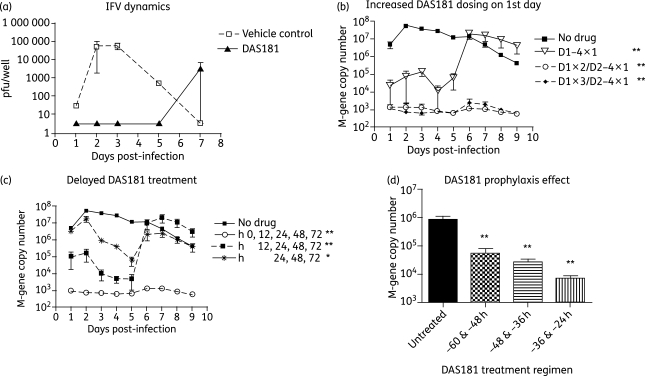
Figure 4.
Antiviral activity of DAS181 in HAE culture. To determine the dynamics of IFV growth and drug effect, PBS or DAS181 (153 µg/cm2) was incubated with HAE tissues for 2 h, followed by infection with IFV A/PR8/34 (5000 pfu/well). Apical washes from the HAE were collected daily and analysed by plaque assay (a). To determine the effect of increased dosing in the first day, HAE cultures were first infected with IFV A/PortChalmers/1/73 (10 000 pfu/well) and then treated with DAS181 daily (D1–4 × 1), twice in the first day and then daily (D1 × 2/D2–4 × 1) or three times on the first day and then daily (D1 × 3/D2–4 × 1). All treatment regimens lasted 4 days and were initiated immediately post-infection (b). To assess the relative benefit of delayed treatments, HAE cultures were infected with IFV A/PortChalmers/1/73 as above and then treated with DAS181 once daily beginning at 0, 12 or 24 h post-infection as indicated. All treatment regimens were maintained for 4 days post-infection (c). To assess the benefit of prophylaxis treatments, HAE cultures were given two DAS181 treatments (12 h apart) beginning at 60, 48 or 36 h pre-infection as indicated and then infected with IFV A/PortChalmers/1/73 as above (d). In (b), (c) and (d) all DAS181 treatments were at 10 µg/cm2 for 2 h followed by washing out of the drug. Apical washes were collected daily and analysed by qRT–PCR at days 1–9 post-infection (b and c) or at day 1 post-infection (d). Values represent means ± SEM of duplicate or triplicate samples. The limit of detection in the plaque assay was 4 pfu/well and the limit of detection in the qRT–PCR assay was 103 M-gene copies. *P < 0.05, **P < 0.01, significantly different for days 1–5 compared with untreated controls (ANOVA with Bonferroni post-test).
Antiviral activity in HAE
In dose–response studies (Table 1) HAE cultures were pre-incubated with DAS181 (2 h at 37°C, followed by two PBS washes) and then infected with various IFVs (5000–40 000 pfu/well). Cultures were washed apically with 100 µL of PBS daily to roughly mimic mucociliary clearance. Apical wash was collected on day 3 for analysis of viral titre via plaque assay on MDCK cells,4 or qRT–PCR analysis of viral M-gene copy number.
Table 1.
Broad-spectrum DAS181 anti-IFV effect in HAE
| Effective concentration of DAS181, μg/cm2 |
||||
|---|---|---|---|---|
| IFV strains | Infection level (pfu/well) | EC50 | EC90 | EC99 |
| Analysed by plaque assay | ||||
| A/PR8/34 (H1N1) | 5000 | 9.24 | 11.29 | 15.09 |
| A/PortChalmers/1/73 (H3N2) | 5000 | 4.83 | 8.39 | 11.13 |
| B/Maryland/1/59 | 5000 | <0.25 | 1.03 | 10.50 |
| Analysed by qRT–PCR | ||||
| A/Weiss/43 (H1N1) | 10 000 | 0.27 | 0.83 | 2.02 |
| A/Victoria/3/75 (H3N2) | 10 000 | 15.51 | 23.82 | 36.73 |
| A/PortChalmers/1/73 (H3N2) | 20 000 | 7.97 | 13.83 | 29.53 |
| B/Maryland/1/59 | 20 000 | 3.03 | 30.51 | 101.50 |
| A/HongKong/2637/04 (clinical) | 40 000 | 11.42 | 18.64 | 28.02 |
DAS181 was incubated with HAE tissues for 2 h immediately prior to IFV infection. Viral loads in the supernatant of the infected wells at day 3 post-infection were determined by plaque assay or qRT–PCR and plotted against DAS181 concentration to determine EC50, EC90 and EC99. Values represent means of duplicate or triplicate samples.
Antiviral mechanism of action
Various concentrations of DAS181, DAS180 and DAS185 were incubated with HAE tissues for 2 h at 37°C followed by two PBS washes, before infection with IFV A/PortChalmers/1/73. After washing out unbound IFV the cultures were replenished with fresh DAS daily (each treatment being 2 h at 37°C, followed by two PBS washes). Day 3 apical washes for all concentrations were analysed by qRT–PCR to determine dose–response.
Dose regimen analysis in HAE
In Figure 4(b) HAE cultures were infected with IFV A/PortChalmers/1/73 and then treated with DAS181 (all treatments 10 µg/cm2 for 2 h) starting immediately post-infection. Cultures were treated for 4 days post-infection with variations in the number of treatments in the first day; either treated once in the first day (D1–4 × 1 = 0, 24, 48 and 72 h), twice in the first day (D1 × 2/D2–4 × 1 = 0, 12, 24, 48 and 72 h) or three times in the first day (D1 × 3/D2–4 × 1 = 0, 8, 16, 24, 48 and 72 h). Daily apical washes were collected and analysed by qRT–PCR (days 1–9).
For the delayed treatment studies (Figure 4c) the HAE cultures were first infected with IFV A/PortChalmers/1/73 and then treated daily with DAS181 (all treatments 10 µg/cm2 for 2 h) starting at various times post-infection (0, 12 and 24 h). All regimens were carried out through 4 days post-infection, and resumed daily treatment at 24 h. Daily apical washes were collected and analysed by qRT–PCR (days 1–9).
For the prophylaxis studies (Figure 4d) the HAE culture were first treated with DAS181 (two treatments separated by 12 h, each at 10 µg/cm2 for 2 h) and then infected with IFV A/PortChalmers/1/73 after various incubation times to enable sialic acid regeneration. Treatment regimens involved treatments at 60 and 48 h pre-infection, 48 and 36 h pre-infection, and 36 and 24 h pre-infection. The apical wash from day 1 (24 h post-infection) was collected and analysed by qRT–PCR.
De-/resialylation and anti-IFV studies with ex vivo human bronchi tissue sections
For testing DAS181 dose levels, the tissue fragments (0.25 cm2) were treated with 0.05 mL of DAS181 solutions at ∼25 µg/mL (5 µg/cm2), 50 µg/mL (10 µg/cm2) and 250 µg/mL (50 µg/cm2) for 2 h at 37°C, before washing, fixation, sectioning and staining with digoxigenin-labelled SNA lectin (Dig Glycan Detection Kit, Roche), which recognizes α2,6-linked sialic acids according to previously described methods.11 As the MAA (Maackia amurensis) lectin identifies non-sialic acid this was not used to determine desialylation effects. Images were detected with a Nikon Eclipse 80i microscope.
For testing the desialylation rate, the tissue fragments were incubated with 0.05 mL of DAS181 solutions at ∼25 µg/mL (5 µg/cm2), 50 µg/mL (10 µg/cm2) and 250 µg/mL (50 µg/cm2) for various time periods of 10, 30 or 60 min at 37°C, followed by fixation and SNA binding. For testing the resialylation rate, the tissue sections were treated with 0.05 mL of DAS181 at 50 µg/mL (10 µg/cm2) for 2 h followed by different incubation times (0, 24, 48 or 72 h) before fixation and SNA staining.
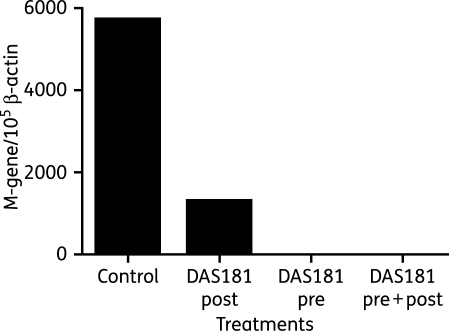
For IFV infection studies, the tissue fragments were infected with IFV for 1 h then washed. Tissues were treated with DAS181 (2 h exposure at 10 µg/cm2) immediately pre-infection (‘pre’), at 24 h post-infection (‘post’) or both (‘pre + post’). After all treatments the tissues were washed with PBS. At 48 h post-infection the tissues were homogenized with a Qiagen tissue homogenizer and RNA was extracted for infectious viral titre determination by qRT–PCR (M-gene copy number normalized to β-actin copy number).
Statistical analysis
In all cases statistical significance was determined using analysis of variance (ANOVA) with Bonferroni post-test, and is described in detail in each figure legend. In Figure 4(b and c) significance is determined for the values through day 5 only, since this represents the period with treatment effect.
Results
Desialylation of HAE culture and ex vivo human bronchial tissue
We have previously shown that DAS181 effectively removes α(2,6)-linked and α(2,3)-linked sialic acids on the MDCK cell surface.4 Here we sought to investigate the DAS181 removal of sialic acid from HAE cultures, which closely mimic the in vivo environment of the human airway. In humans, the epithelial lining of the trachea, bronchi and bronchioles exhibits abundant α(2,6)-linked sialic acid, the receptor for all human IFV strains, while the level of α(2,3)-linked sialic acid, the receptor used by avian IFV strains, is barely detectable.12 We observed the same pattern of sialic acid distribution on the apical surface of HAE. After a 2 h treatment by DAS181 over a range of doses, the level of α(2,6)-linked sialic acid was reduced in a dose-dependent manner (Figure 1a). Because the level of α(2,3)-linked sialic acid in the untreated HAE is barely detectable, the difference in α(2,3)-linked sialic acid level on the HAE surface after DAS181 treatment was not perceptible (data not shown).
Two DAS181 analogues, DAS180 and DAS185, were also tested for desialylation of HAE. Compared with DAS181, DAS180 lacks the heparin-binding domain (AR domain) that prolongs drug retention on the airway epithelium; DAS185 is inactive in sialidase function due to a point mutation (Y348F) in the sialidase active domain.4 As expected, DAS180 desialylated the HAE, although its desialylation potency as measured by EC50 (50% effective concentration) is >20-fold weaker than that of DAS181, whereas DAS185 failed to desialylate the epithelium (data not shown). These results confirmed that sialidase activity is critical for DAS181 function and the AR domain significantly enhances DAS181 function on the airway epithelium.
Next, we investigated the relationship between DAS181 concentration and duration of exposure in terms of sialic acid removal. Different DAS181 exposure times to HAE (0.25, 2, 6 and 24 h) were tested, and the result demonstrated that the desialylation effect is a function of both DAS181 local concentration and duration of exposure. While at low DAS181 concentrations, the time of exposure was most critical, as the concentration reached a level of ≥30 µg/cm2, nearly 90% desialylation was achieved in 15 min, and longer treatment duration did not appear to make a significant difference (Figure 1b). At a concentration of 8 µg/cm2, ∼80% desialylation was achieved in 2 h (Figure 1b).
Similar to the airway epithelium in vivo, HAE produces mucus. To investigate if the presence of mucus would interfere with DAS181 function, DAS181 was added either to the fresh HAE with the mucus layer intact or to the HAE extensively washed with PBS, and desialylation activity under both conditions was compared. As shown in Figure 1(c), the presence of the mucus layer on HAE did not affect the ability of DAS181 to remove cell surface sialic acids, as revealed by essentially identical desialylation dose–response curves with or without mucus.
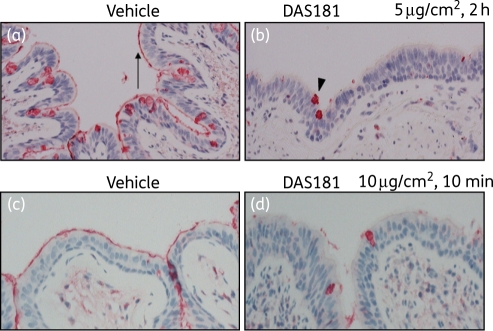
DAS181 function was next evaluated in the fresh ex vivo human bronchial tissue model. The bronchial tissue was obtained from patients undergoing bronchial resection. It was immediately sectioned (without washing in order to preserve the endogenous mucus), treated with DAS181, washed and then monitored for desialylation or resialylation on the apical surface of the cultured tissue at various timepoints. In the first experiment, the bronchial tissue sections were cut into small fragments and incubated with DAS181 at three concentrations (0, 5, 10 or 50 µg/cm2) for 2 h, followed by fixation and sialic acid detection. At concentrations as low as 5 µg/cm2 DAS181 appeared to fully desialylate the apical surface of bronchial tissue in 2 h (Figure 2a and b), consistent with the observation with HAE. No further desialylation was evident with doses >5 µg/cm2 (data not shown). It was noted that while the microvilli on the apical cell surface were extensively desialylated (arrow), the intracellular sialic acid present in goblet cells was not removed, persisting as intracellular globules (arrowhead). This result is to be expected since DAS181 functions primarily on the cell surface.
Figure 2.
DAS181 effectively removes sialic acid from ex vivo human bronchi tissue. Fresh human bronchi sections were treated with vehicle (a) or DAS181 at 5μg/cm2 (b) for 2 h at 37°C, washed, fixed and then sectioned before immediate detection of α2,6-sialic acid levels with lectin SNA. In separate experiments fresh human bronchi sections were treated with vehicle (c) or DAS181 at 10μg/cm2 (d) for 10 min at 37°C, followed by fixation and detection of α2,6-sialic acid levels as above. Red/dark staining represents sites of high α2,6-sialic acid levels. The arrow in (a) shows the epithelial surface of ciliated cells appearing as a red/dark line, and the arrowhead in (b) shows the intracellular mucin of goblet cells appearing as a red/dark globule. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
The second experiment aimed to examine the desialylation rate of DAS181 at different concentrations (0, 5 or 10 µg/cm2), in which the tissue sections were treated for time periods of 10, 30 or 60 min, followed by fixation and the detection of the remaining sialic acid with lectin SNA. The result revealed that at both 5 and 10 µg/cm2 DAS181 treatment as short as 10 min significantly removed the apical surface sialic acid on bronchial tissues (Figure 2c and d). The full extent of desialylation appeared to be achieved at the 10 µg/cm2 dose level in 10 min and no further desialylation was evident at the later timepoints (data not shown). It was noted that the 5 µg/cm2 dose at 10 min still showed a mild degree of SNA binding compared with the 10 µg/cm2 dose, but that difference was eliminated by 30 min (data not shown). This result is consistent with the findings in the HAE cultures and confirms that 5–10 µg/cm2 represents the range of the minimal local level of DAS181 to significantly desialylate the airway epithelial surface.
Sialic acid regeneration following DAS181 treatment of HAE culture and ex vivo human bronchial tissue
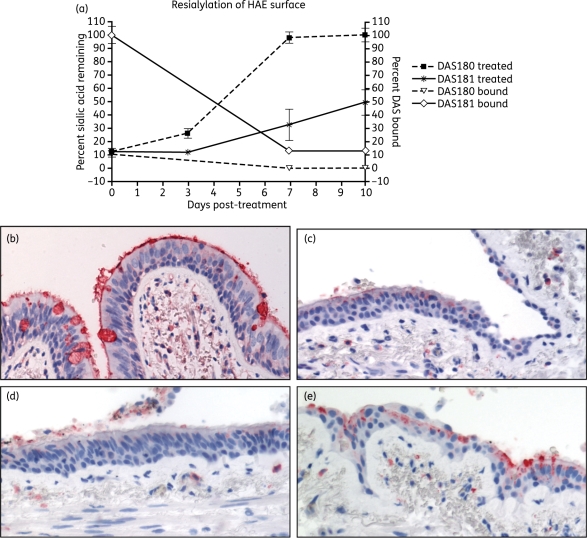
In MDCK cells, after DAS181 treatment the surface sialic acid remained low and unchanged for 48 h but rebounded in 80 h.4 To assess the rate of sialic acid regeneration after DAS181 treatment in HAE, the HAE cultures were washed and incubated for 10 days following a single 2 h DAS181 treatment at 7.7 µg/cm2. During the incubation, the cultures were washed once daily to partially mimic the in vivo mucociliary clearance effect. At various timepoints a subset of the cultures were fixed and processed to determine the remaining DAS181-bound and α(2,6)-linked sialic acid levels. The results showed that a single treatment reduced sialic acid by ∼90%, and this reduced level was maintained for at least 3 days. By day 7 post-treatment a minor increase in surface sialic acid (∼30% of control) was detected, followed by a slow and incomplete recovery to ∼50% of control on day 10 post-treatment (Figure 3a). The observed slow regeneration of sialic acid correlated to the long retention of DAS181 on the HAE surface and indeed ∼15% of the cell-bound DAS181 was still present after day 7 post-treatment (Figure 3a). In contrast, the level of cell-bound DAS180 was only 10% of that for DAS181 immediately after treatment and DAS180 quickly became undetectable during incubation (Figure 3a). As a result, although DAS180 treatment caused a level of initial desialylation identical to DAS181 (∼90% reduction), the HAE resialylation occurred faster and to a greater extent (∼25% on day 3 and ∼100% on day 7) than that of DAS181 (Figure 3a).
Figure 3.
Time course of sialic acid regeneration on HAE and ex vivo human bronchi following DAS181 treatment. HAE cultures were incubated with DAS180 or DAS181 at 7.7μg/cm2 for 2 h at 37°C followed by incubation for 10 days with daily PBS washing to mimic mucociliary clearance. At selected timepoints surface levels of α2,6-sialic acid or DAS protein were determined (a). In corroboratory experiments fresh human bronchi sections were treated with vehicle (b) or DAS181 at 10μg/cm2 (c–e) for 2 h at 37°C, washed and then incubated in medium for 24, 48 or 72 h, before detecting levels of α2,6 sialic acid. Red/dark staining represents sites of high α2,6-sialic acid levels. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
We also tested the rate of ex vivo bronchi tissue resialylation following a single 2 h DAS181 exposure (10 µg/cm2). The tissue sections were treated with DAS181 followed by washing and incubation in medium for 0, 24, 48 or 72 h before fixation. The result showed that the DAS181-induced desialylation of the bronchial epithelium was maintained for at least 48 h after the DAS181 treatment, but a low level of resialylation started to appear at 72 h post-treatment (Figure 3b–e).
IFV infectious course in HAE
Unlike in MDCK cells, in the HAE cultures IFV does not require exogenous trypsin to replicate to high titres. In general, depending on the IFV strain, after inoculating a challenge dose of IFV (5000–10 000 pfu/well in a 96-well plate) to the HAE apical surface on day 0, virus shedding, as quantified by plaque assay, reaches peak levels around day 2 or day 3. Afterwards, it gradually declines and becomes undetectable between day 7 and day 12 (Figure 4a and b and Figure S1). The termination of IFV shedding in HAE is caused by a lack of susceptible cells at the end of the IFV infectious course.
To increase throughput, we also quantified virus titre by an RT–PCR method based on quantitative detection of the IFV M-gene. Compared with the plaque assay analysis the RT–PCR method revealed identical HAE dynamics of IFV infection initiation and peak, but at the later phase of infection the decline of IFV infection seemed to be slower (Figure S1). Thus, while the RT–PCR method is comparable to the plaque assay for demonstrating initiation and peak of IFV infection in the HAE system, it is less indicative of the rate of decline in viral shedding during the resolution phase of the infectious course.
Inhibition of IFV replication in HAE
To assess the anti-IFV activity of DAS181, HAE cultures were pre-treated with various concentrations of DAS181 and then infected with different strains of IFV and at different challenge doses. The cultures were washed daily to partially mimic mucociliary clearance. On day 3 post-infection the apical wash was collected for viral titre quantification by either plaque assay (pfu/mL) or RT–PCR assay. Results from both assays demonstrated the potent anti-IFV effect of DAS181 (Table 1). Judged by the EC50, EC90 and EC99 values, DAS181 at a local concentration of ≥10 µg/cm2 confers significant anti-IFV inhibition in the HAE model system. This concentration of DAS181 correlates to ∼80% desialylation of the HAE surface (Figure 1b).
IFV infection causes host cell apoptosis, mediated by the changes in the expression of the genes involved in apoptosis pathways.13,14 We investigated these changes using an expression array consisting of detection of 84 key apoptosis-related genes. HAE culture infected with IFV B/Maryland/1/59 revealed several changes in apoptosis-related gene expression, including strong upregulation of BIK (BCL2-interacting killer; pro-apoptotic gene) and downregulation of TNFRSF11b (tumour necrosis factor receptor superfamily member 11b, also known as osteoprotegrin; anti-apoptotic gene) and BAG4 (BCL2-associated athanogene 4; anti-apoptotic gene) (Table 2). However, pre-treatment with DAS181 at 7.5 µg/cm2 for 2 h completely suppressed the expression changes of the three apoptosis genes induced by IFV infection, thus protecting host cells from viral-induced apoptosis.
Table 2.
Suppression of IFV-induced apoptosis in HAE by DAS181
| Fold change in gene expression |
||
|---|---|---|
| Apoptosis gene | PBS treated | DAS181 treated |
| BIK | 13.7 | 1.1 |
| BAG4 | −475.0 | −1.4 |
| TNFRSF11b | −898.9 | −1.2 |
PBS or DAS181 (7.5 µg/cm2) was incubated with HAE tissues for 2 h immediately prior to IFV B/Maryland/1/59 infection (10 000 pfu/well). Cellular RNA was later extracted and assayed for apoptosis gene expression. Fold changes of gene expression, from untreated HAE culture, are shown for the genes with changes ≥2.0-fold.
As expected from their distinct desialylation functions, DAS181 and its analogues, DAS180 and DAS185, also differ in their anti-IFV functions. While only DAS181 and DAS180, not DAS185, inhibited IFV infection in HAE, DAS181 was >3-fold more potent than DAS180 as measured by EC99, which highlights the role of the AR tag in improving DAS181 potency (data not shown).
DAS181 treatment regimen analysis in HAE
The HAE system differs from the human respiratory epithelium in that it lacks the innate and the adaptive immune mechanisms that are responsible for the virus control and clearance in vivo. As a result, it is to be expected that after being initially inhibited by DAS181, residual IFV may eventually break through and infect HAE as the DAS181 treatment effect wanes over time. In the case of IFV A/PR8/34 infection of HAE, a single high-dose level treatment of DAS181 applied prior to infection abrogated infection for at least 5 days, and the breakthrough virus was detected on day 7 (Figure 4a). We have demonstrated that the breakthrough virus in HAE did not exhibit meaningful resistance to DAS181 (data not shown), and thus instead resulted from the diminished DAS181 treatment effect after day 5. It should also be noted that in the environment of the human respiratory tract in vivo, any significant delay of the virus replication will aid the host immune system to fight against the virus, and thus will lead to certain therapeutic benefit to the patients.
To assess the DAS181 treatment regimen, DAS181 at the suggested therapeutic range (10 µg/cm2, 2 h) was applied at various frequencies to the HAE cultures after inoculation of IFV A/PortChalmers/1/73 (H3N2). A once-a-day treatment regimen given for 4 days (D1–4 × 1) inhibited viral titre by up to >3 logs. After the last treatment was given on day 3, the viral titre remained low for two more days and rebounded on day 6 (Figure 4b). Interestingly, a regimen that has one additional treatment in the first 24 h post-infection (D1 × 2/D2–4 × 1) completely inhibited the initial infection as well as the subsequent viral breakthrough during the 9 day incubation (Figure 4b). No additional benefit was observed when adding a third treatment on day 0 (D1 × 3/D2–4 × 1). This result indicates that a higher loading dose in a post-infection treatment regimen may bring significant therapeutic benefit.
In the next experiment, DAS181 treatment was initiated at various timepoints after IFV infection of the HAE. As a control, DAS181 (10 µg/cm2, 2 h) was given immediately after IFV inoculation using the most effective 4 day regimen based on the result described above (D1 × 2/D2–4 × 1), and IFV replication was completely inhibited (Figure 4c). In the next treatment group, a similar, but shorter, 3 day DAS181 regimen (D1 × 2/D2–3 × 1) was initiated at 12 h post-infection. IFV replication was stalled and reduced by up to 4 logs for ∼5 days, but the virus breakthrough occurred on day 6 (Figure 4c). Interestingly, even when initiated at 24 h post-inoculation, as IFV infection approaches the peak level, DAS181 in a minimal treatment regimen (D2–4 × 1) still inhibited IFV infection by up to >2 logs in the HAE, and the treatment effect lasted for at least 2 days after the last dose (Figure 4c).
To demonstrate prophylaxis protection against IFV by DAS181, HAE cultures were treated with two doses of DAS181 (10 µg/cm2, 2 h) given 12 h apart, with the second treatment ending at 24, 36 or 48 h prior to viral inoculation at high dose (10 000 pfu/well). As judged by viral titre at 24 h post-infection, DAS181 significantly reduced IFV infection in all of the prophylactic treatment regimens even when the last treatment was applied at 48 h prior to the challenge (Figure 4d).
IFV inhibition by DAS181 in ex vivo culture of human bronchial tissue sections
To confirm the anti-IFV effect of DAS181 observed in the HAE cultures, we next infected and treated fresh human bronchial tissue sections. Upon infection by A/HK/54/98 (H1N1), a high level of virus was detected by RT–PCR in the untreated bronchial tissue washes at 48 h after viral challenge. In the bronchial tissue treated with a single dose of DAS181 (2 h at 10 µg/cm2) immediately prior to the virus challenge, the viral infection was completely abrogated. When a single dose of DAS181 was instead given at 24 h post-infection, a significant reduction in viral infection was still observed (Figure 5).
Figure 5.
Effective inhibition of IFV infection in ex vivo human bronchi by DAS181. Fresh human bronchi sections were infected with A/HK/54/98 (H1N1) and treated with PBS (control) or DAS181 immediately pre-infection (‘pre’), at 24 h post-infection (‘post’) or both (‘pre + post’). Tissues were washed after all DAS181 treatments (2 h at 37°C, 10 µg/cm2). Tissues were incubated for 48 h before homogenization and determination of viral M-gene copy number by qRT–PCR. M-gene copy numbers were normalized to cellular β-actin copy number and are plotted as M-gene copy number per 105 β-actin copy number.
Discussion
In this report, the anti-IFV pharmacodynamics of DAS181 were evaluated in two model systems: the HAE culture and the fresh human bronchial tissue section. These two model systems represent superior simulators of the human respiratory tract compared with the commonly used MDCK cells because they carry the same cell type composition and polarity, mucus-secreting function and mucociliary movements as the airway epithelium in vivo. In addition, the composition and expression level of the sialylated glycans in vivo are expected to be well duplicated in these models. Thus they are relevant in vitro models for investigating the dynamics of desialylation/resialylation and anti-IFV activity. Importantly, the dynamics of IFV infection in HAE shown here are very similar to the previously reported IFV infectious course in a human challenge study.15
Here we show that DAS181 desialylates HAE culture and ex vivo bronchi tissue and therefore inhibits infection by several IFV strains. Study results have revealed that ∼80% epithelial surface desialylation and significant anti-IFV efficacy can be achieved at a topical concentration of DAS181 in the range of 5–10 µg/cm2 when applied once daily. Furthermore, an additional treatment (or a higher loading dose) on the first day brings significant additional treatment benefit in these model systems. Extensive studies with HAE culture have not indicated any cytotoxic affect of DAS181 treatment as high as 1300 µg/cm2 [visual observation, MTS/LDH assay, apoptotic gene expression microarray and numerous animal studies have found DAS181 treatment to the respiratory tract to be well tolerated (L. M. Aschenbrenner, E. R. Campbell, J. M. Nicholls and F. Fang, unpublished data)].
The rate of sialic acid turnover on the airway epithelium has never been characterized. Previously the cell surface sialic acids on a T cell lymphoma cell line were shown to be primarily on glycoproteins (93%), and resialylation of the cell surface to be mostly contingent upon de novo protein synthesis.16 Data from the ex vivo human bronchial tissue suggest that desialylation and the anti-IFV effect from a single DAS181 treatment would last for at least 2 days. Thus the sialic acid regeneration on the airway epithelial surface, even in the presence of IFV infection, may take longer than 48 h. Consistently IFV inhibition by DAS181 in HAE lasted for at least 2 days following the final treatment in various pre-infection or post-infection treatment regimens. The sialic acid regeneration following DAS181 treatment in the uninfected HAE cultures, however, seems to take longer than 3 days. The apparent longer duration for sialic acid regeneration in normal HAE cultures may be due to a combined effect of slower protein synthesis and a lack of mucociliary cleaning that results in longer DAS181 retention in the uninfected HAE.
The lack of any innate or adaptive immune mechanisms for viral control and clearance in HAE makes this model system very stringent for assessing antiviral efficacy of a drug candidate. To this end, ex vivo human bronchial tissue may provide more accurate assessment of potential drug efficacy as these tissues may contain some macrophages and cytotoxic T lymphocytes that can secrete interferon or kill the infected cells.17–19 Importantly, the purported functions of DAS181 are verified here in the human bronchial tissue sections, and in addition DAS181 appears to be more potent when applied to the bronchial tissue than the HAE cultures.
To date, in vitro efficacies of anti-IFV drug candidates are almost exclusively derived from studies using non-polarized cell line monolayers. Only one NAI, oseltamivir carboxylate, was reported to be effective in HAE against IFV.20 In the report, oseltamivir efficacy was assayed by the number of infected cells. Whereas 47-fold fewer cells were infected when the drug was added shortly before the infection, a 1 h delay in treatment resulted in only 1.5-fold inhibition. Additionally, if the drug was given at 4 h after virus inoculation, no inhibition was observed.20 These results are in stark contrast to the potent anti-IFV effect of DAS181 presented in this report. DAS181 compares very favourably against oseltamivir in the HAE model system; in particular, DAS181 inhibited IFV by >2 logs even when it was given at 24 h after virus inoculation.
Dynamic models of IFV infection in humans have revealed that the respiratory tract is not only the site of infection but also the site of defence by the host. In a natural infectious course, during the first 3–5 days after the primary infection, the magnitude of infection expands rapidly until the total infected cell number, virus shedding and symptoms reach the peak level. At this stage, severity of the infection is primarily controlled by the number of susceptible cells, and not by the adaptive anti-IFV immune response.21–23 During a natural infection, DAS181 treatment may achieve two effects: (i) by reducing the magnitude of infection, clinical symptoms may be ameliorated; and (ii) by inhibiting viral replication for days, DAS181 may allow the adaptive immune response enough time to develop and eliminate the virus.
Blocking viral binding to host cell receptors is an attractive antiviral approach. It is well established that both IFV and human parainfluenza virus (HPIV) utilize cell surface sialic acid for binding and entry to initiate viral infection. A previous report described desialylation and anti-HPIV-3 activity of the sialidase from Vibrio cholerae, but the sialidase failed to protect HAE against infection by the IFV A/England/26/99 strain, probably due to incomplete desialylation.24 DAS181 has previously demonstrated anti-IFV activity in vitro using monolayer cell cultures, in vivo using mice and ferrets, and in ex vivo human lung tissue,4,5,25 the results presented here are in support of these previous findings. DAS181 is also effective at inhibiting various HPIV strains both in vitro and in vivo (data not shown). Ultimately, the merits of a drug candidate need to be assessed by testing in humans. The safety and efficacy of DAS181 are currently being evaluated in clinical trials.
Funding
The work at NexBio was supported in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant numbers U01AI070281 and R44AI056786, and contract number HHSN266200600015C). The work at the University of Hong Kong was supported by funding from: the Research Fund for the Control of Infectious Diseases (grant number 08070842); Competitive Earmarked Research Grants (grant number 773507M); and the Area of Excellence programme on Influenza supported by the University Grants Committee of the Hong Kong Special Administrative Region, China (Project number AoE/M-12/06).
Transparency declarations
G. B. T.-B., L. M. A., E. R. C., M. B., Q.-X. L. and F. F. are/were employees of NexBio, a developer of DAS181, and declare they have competing financial interests including ownership of stock or options in the company. J. M. N., R. W. Y. C., J. S. M. P., M. C. W. C. and A. C. N. W. declare that they have no competing financial interests.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Supplementary Material
Acknowledgements
We thank David Wurtman and Ronald Moss for critical review of this manuscript prior to submission.
References
- 1.Englund JA. Antiviral therapy of influenza. Semin Pediatr Infect Dis. 2002;13:120–8. doi: 10.1053/spid.2002.122999. [DOI] [PubMed] [Google Scholar]
- 2.Reece PA. Neuraminidase inhibitor resistance in influenza viruses. J Med Virol. 2007;79:1577–86. doi: 10.1002/jmv.20951. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls JM, Chan RW, Russell RJ, et al. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008;16:149–57. doi: 10.1016/j.tim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–9. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belser JA, Lu X, Szretter KJ, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196:1493–9. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–7. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls JM, Chan MC, Chan WY, et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13:147–9. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- 8.Ward CL, Dempsey MH, Ring CJ, et al. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J Clin Virol. 2004;29:179–88. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Liu B, Maminishkis A, et al. Gene expression profiling in autoimmune noninfectious uveitis disease. J Immunol. 2008;181:5147–57. doi: 10.4049/jimmunol.181.7.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls JM, Aschenbrenner LM, Paulson JC, et al. Comment on: Concerns of using sialidase fusion protein as an experimental drug to combat seasonal and pandemic influenza. J Antimicrob Chemother. 2008;62:426–8. doi: 10.1093/jac/dkn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinya K, Ebina M, Yamada S, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 13.Brydon EW, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev. 2005;29:837–50. doi: 10.1016/j.femsre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Lowy RJ. Influenza virus induction of apoptosis by intrinsic and extrinsic mechanisms. Int Rev Immunol. 2003;22:425–49. doi: 10.1080/08830180305216. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichner JS, Whiteheart SW, Hart GW. Intracellular trafficking of cell surface sialoglycoconjugates. J Biol Chem. 1988;263:16316–26. [PubMed] [Google Scholar]
- 17.Gong JH, Sprenger H, Hinder F, et al. Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-alpha (TNF-alpha) gene expression and lipopolysaccharide-triggered TNF-alpha release. J Immunol. 1991;147:3507–13. [PubMed] [Google Scholar]
- 18.Matsukura S, Kokubu F, Noda H, et al. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98:1080–7. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 19.Peschke T, Bender A, Nain M, et al. Role of macrophage cytokines in influenza A virus infections. Immunobiology. 1993;189:340–55. doi: 10.1016/s0171-2985(11)80365-7. [DOI] [PubMed] [Google Scholar]
- 20.Matrosovich MN, Matrosovich TY, Gray T, et al. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78:12665–7. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bocharov GA, Romanyukha AA. Mathematical model of antiviral immune response. III. Influenza A virus infection. J Theor Biol. 1994;167:323–60. doi: 10.1006/jtbi.1994.1074. [DOI] [PubMed] [Google Scholar]
- 22.Chang DB, Young CS. Simple scaling laws for influenza A rise time, duration, and severity. J Theor Biol. 2007;246:621–35. doi: 10.1016/j.jtbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Hancioglu B, Swigon D, Clermont G. A dynamical model of human immune response to influenza A virus infection. J Theor Biol. 2007;246:70–86. doi: 10.1016/j.jtbi.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CI, Barclay WS, Zambon MC, et al. Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol. 2006;80:8060–8. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan RW, Chan MC, Wong AC, et al. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53:3935–41. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.