Abstract
OBJECTIVE
It is believed that disruption of vulnerable atherosclerotic plaque and subsequent thrombus formation play critical roles in the pathogenesis of cerebral infarction. We simultaneously determined four relatively common genetic variants related to plaque rupture or subsequent local thrombus formation and evaluated the combined effect on cerebral infarction.
RESEARCH DESIGN AND METHODS
We enrolled 3,094 Japanese type 2 diabetic subjects (62.7% male; aged 61.5 ± 8.4 years) and determined their genotypes regarding matrix metalloproteinase 9 C-1562T, coagulation factor XII (F12) C46T, von Willebrand factor (VWF) G-1051A, and plasminogen activator inhibitor (PAI-1) 675 4G/5G polymorphisms. The diagnosis of cerebral infarction was performed based on history, physical examination, and neuroimaging.
RESULTS
The single association analysis revealed that there were no statistically significant associations between each polymorphism and the prevalence of cerebral infarction. Interestingly, the prevalence of cerebral infarction was higher with the increase of the total number of four concomitant unfavorable proatherothrombotic alleles in each subject (P value for linear trend = 0.004). Furthermore, a multiple logistic regression analysis showed that the number of proatherothrombotic alleles was a risk factor for cerebral infarction independently of conventional risk factors (odds ratio for one-point increase in the number of proatherothrombotic allele 1.15 [95% CI 1.05–1.26], P = 0.004).
CONCLUSIONS
Accumulation of gene polymorphisms related to plaque rupture and thrombus formation is likely associated with the prevalence of cerebral infarction in type 2 diabetic patients, suggesting that the combined information about these variants is useful to assess the risk of cerebral infarction.
Cerebral infarction, a major cause of severe disability and death in subjects with type 2 diabetes, is caused by focal cerebral ischemia due to arterial occlusion. Various pathophysiological mechanisms can result in cerebral infarction, but the most common one is thrombus formation in preexisting atherosclerosis. After disruption of vulnerable atherosclerotic plaque and exposure of prothrombotic subendothelial matrix, local thrombus formation occurs, which subsequently leads to occlusion of cerebral arteries. Thus, the pathogenesis of cerebral infarction is complex and involves multiple genetic and environmental factors related to atherogenesis and thrombosis (1,2).
Several molecules are reported to play key roles in the plaque rupture and subsequent local thrombus formation. Matrix metalloproteinase (MMP) 9 (gelatinase B), a 92-kDa type IV collagenase, is an enzyme with proteolytic activity against connective tissue proteins such as collagens, proteoglycans, and elastin. Since MMP9 affects the volume expansion of the atherosclerotic plaque, its stability, and the potential for smooth muscle cell proliferation by regulating connective tissue remodeling, it is likely implicated in the pathophysiology of atherogenesis and plaque rupture, the most common mechanism of atherothrombosis (3–5). Functional studies indicate that the −1562C>T polymorphism has an allele-specific effect on MMP9 transcription and that MMP9 protein levels and activity were higher in the −1562T allele carriers (6,7).
Coagulation factor XII (F12; Hageman factor), an 80-kDa serine protease circulating in plasma, plays a central role in the initiation of coagulation. In some studies, high F12a levels have been reported in arterial thrombo-embolic disease (8,9). A C to T substitution at nucleotide 46 in the 5′ untranslated region of the F12 gene destroys a Kozak consensus sequence, resulting in lower translation efficiency and decreased F12a plasma levels (10,11). von Willebrand factor (VWF), a large circulating glycoprotein mainly derived from vascular endothelium, also plays a pivotal role in platelet adhesion and aggregation at sites of high shear rates such as in arteries with stenotic or ruptured atherosclerotic plaque lesions. Elevated plasma VWF levels are associated with cardiovascular disease (CVD), and the plasma VWF level has been established as an independent predictor of cardiovascular risk (12). It has been reported that three linked dimorphisms (−1,234C/T, −1,185A/G and −1,051G/A) in the VWF gene promoter influence plasma VWF level; individuals with the CC/AA/GG genotype have the highest mean VWF levels (13).
Plasminogen activator inhibitor (PAI-1) is one of the central components of the fibrinolytic system. A deletion/insertion (4G/5G) polymorphism at −675 bp upstream from the mRNA initiation point in the promoter lesion of PAI-1 gene is associated with plasma PAI-1 levels. The 4G allele is associated with higher levels of gene transcription and elevated PAI-1 plasma levels compared with 5G counterpart and thereby possibly increases the risk for intravascular thrombosis (14).
Although there have been studies on the associations between each polymorphism and CVD, such as coronary artery disease (CAD) and cerebral infarction, the results of these studies remain controversial (11,15–22). Since individual genetic polymorphism generally confers only moderate atherosclerotic risk at most, the determination of each polymorphism may not be enough to assess the risk of CVD. In this study, therefore, we simultaneously determined four relatively common genetic variants related to plaque rupture or subsequent local thrombus formation (MMP9 C-1562T, F12 C46T, VWF G-1051A, and PAI-1 −6754G/5G) and evaluated the combined effect on cerebral infarction.
RESEARCH DESIGN AND METHODS
Japanese type 2 diabetic subjects who periodically attended the outpatient clinics of diabetes in five participating hospitals (Osaka University Medical Hospital, Ehime Prefectural Central Hospital, Ehime Prefectural Imabari Hospital, Ishibashi Clinic, and Naka Kinen Clinic) during 1 year (January 2005 to December 2005) were asked to participate in this study. We considered subjects eligible when they had type 2 diabetes diagnosed by diabetologists and were aged ≥40 years. The determination of type 2 diabetes was based on World Health Organization criteria. After all, a total of 3,094 subjects (62.7% male, [means ± SD] aged 61.5 ± 8.4 years, duration of diabetes 8.0 ± 7.5 years, and A1C 7.1 ± 1.2%) were enrolled.
The study protocol was approved by the committees on the ethics of human research of Osaka University Graduate School of Medicine. A written informed consent was obtained from all the participants after a full explanation of the study.
Clinical and biochemical analysis
Fasting blood samples were collected, and A1C, serum total and HDL cholesterol, and serum triglyceride levels were measured using standard laboratory protocols. The determination of hypertension (defined as systolic blood pressure [SBP] ≥130 mmHg or diastolic blood pressure [DBP] ≥80 mmHg or having been treated for hypertension) and dyslipidemia (defined as serum LDL cholesterol ≥120 mg/dl or serum triglycerides ≥150 mg/dl or HDL cholesterol <40 mg/dl or having been treated for dyslipidemia) was based on the Japan Diabetes Society's criteria. Smoking status was evaluated as follows: a value of 0 and 1 were assigned to subjects when Brinkman's Index (the number of cigarettes per day times smoking years) was <200 and >200, respectively. The diagnosis of cerebral infarction was performed based on history, physical examination, and neuroimaging (computed tomography, magnetic resonance imaging, or both), according to the traditional World Health Organization criteria of stroke. The patients' characteristics and their medication are listed in Table 1.
Table 1.
Patient characteristics
| Sex (female/male) | 1,154/1,940 (37.3/62.7) |
| Age (years) | 61.5 ± 8.4 |
| Duration of diabetes (years) | 8.0 ± 7.5 |
| Smoking status (Brinkman's Index <200/≥200) | 1,572/1,522 (50.8/49.2) |
| BMI (kg/m2) | 24.1 ± 3.5 |
| A1C (%) | 7.1 ± 1.2 |
| Presence of hypertension (%) | 2,352 (76.0) |
| SBP (mmHg) | 133 ± 18 |
| DBP (mmHg) | 80 ± 12 |
| Presence of dyslipidemia (%) | 2,356 (76.1) |
| Total cholesterol (mmol/l) | 4.88 ± 0.78 |
| HDL cholesterol (mmol/l) | 1.48 ± 0.42 |
| Triglycerides (mmol/l) | 1.41 ± 0.98 |
| Treatment approach for diabetes | |
| Diet alone | 541 (17.5) |
| Using oral hypoglycemic agent | 1,793 (58.0) |
| Sulfonylureas | 1,097 (35.5) |
| Thiazolidinediones | 368 (11.9) |
| Biguanides | 1,069 (34.6) |
| Using insulin | 760 (24.6) |
| Treatment approach for dyslipidemia | |
| Using statins | 894 (28.9) |
| Using other drugs | 162 (5.2) |
| MMP9 C-1562T polymorphism | |
| CC genotype (%) | 2,145 (69.3) |
| CT genotype (%) | 867 (28.0) |
| TT genotype (%) | 82 (2.7) |
| F12 C46T polymorphism | |
| CC genotype (%) | 386 (12.5) |
| CT genotype (%) | 1,412 (45.6) |
| TT genotype (%) | 1,296 (41.9) |
| vWF G1051A polymorphism | |
| GG genotype (%) | 985 (31.8) |
| GA genotype (%) | 1,534 (49.6) |
| AA genotype (%) | 575 (18.6) |
| PAI-1 4G/5G polymorphism | |
| 4G4G genotype (%) | 1,245 (40.2) |
| 4G5G genotype (%) | 1,423 (46.0) |
| 5G5G genotype (%) | 425 (13.7) |
| Cerebral infarction (%) | 322 (10.4) |
Data are n (%) or means ± SD.
Genetic analysis
In the present study, four genetic polymorphisms that are potentially associated with plaque disruption and/or thrombus formation (MMP9 C-1562T, F12 C46T, VWF G-1051A, and PAI-1 −6754G/5G) were selected for the analysis. Each allele was determined to be either a proatherothrombotic allele or antiatherothrombotic allele on the basis of prior evidence of potential functionality in plaque rupture and/or thrombus formation. Therefore, the total number of four concomitant unfavorable proatherothrombotic alleles in each subject theoretically ranges from zero (carrying no proatherothrombotic allele) to eight (carrying all of the proatherothrombotic alleles).
Venous blood was collected from each subject and genomic DNA was isolated with a DNA isolation kit (Qiagen). The genotypes of the single nucleotide polymorphisms (SNPs) in each subject were determined with a fluorescence- or colorimetry-based allele-specific DNAprimer probe assay system (Toyobo Gene Analysis) as previously described (23).
Statistical analysis
After each polymorphism was assessed, all the subjects were divided into two or three genotype groups based on the major allele's dominant (AA + Aa genotype versus aa genotype), recessive (AA genotype versus Aa + aa genotype), and additive genetic models (AA genotype < Aa genotype < aa genotype or AA genotype > Aa genotype > aa genotype), and the parameters were compared among the groups. Distributions of continuous variables in groups were expressed as means ± SD. In the dominant and the recessive genetic models, data between two groups were compared by the χ2 test or the Student's t test. In the additive genetic models, the associations between polymorphisms and variables were evaluated with the Mantel extension test or ANOVA with post hoc comparison of the means. Bonferroni's multiple comparison procedure was applied for statistical tests to control the family-wise type 1 error to <0.05 and gave the corrected level of significance, 0.0042 [ = 0.05/(3 × 4)], since multiple comparisons must be performed on the three models of four SNPs.
Multiple logistic regression analyses were performed to evaluate the relationship between the prevalence of cerebral infarction and the following variables: sex, age, smoking status (Brinkman's Index <200 or ≥200), BMI, duration of diabetes, A1C, prevalence of hypertension, prevalence of dyslipidemia, and number of proatherothrombotic alleles. For these, the P value for the inclusion and exclusion of variables was set at 0.05.
RESULTS
Table 1 shows the general characteristics of the subjects. After each of the four polymorphisms (MMP9 C-1562T, F12 C46T, VWF G-1051A, and PAI-1 −675 4G/5G) was assessed, we confirmed that all alleles were in Hardy-Weinberg equilibrium.
First, all subjects were divided into two or three genotype groups based on the major allele's dominant, recessive, and additive genetic models, and the parameters were compared among the groups (supplementary Tables 1–4 [available at http://care.diabetesjournals.org/cgi/content/full/dc09-1518/DC1]). Regarding the PAI-1 −6754G/5G polymorphism, the prevalence of cerebral infarction tended to be higher as the number of proatherothrombotic alleles increased (8.9% in the 5G/5G carriers, 9.5% in 5G/4G carriers, and 12.0% in the 4G/4G carriers; P value for trend = 0.027). Also, the prevalence of cerebral infarction tended to be higher in subjects with the 5G allele than those without it (9.4% and 12.0%, respectively, P = 0.020). However, there was no significant association between this polymorphism and the prevalence of cerebral infarction in any genetic models after Bonferroni's correction was applied (supplementary Table 1). Similarly, regarding MMP9 C-1562T, F12 C46T, and VWF G-1051A polymorphisms, there were no statistically significant associations between these polymorphisms and the prevalence of cerebral infarction (supplementary Tables 2–4).
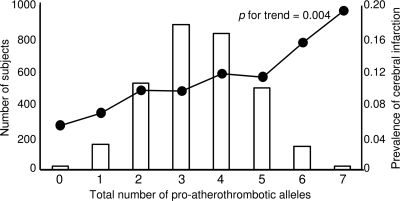
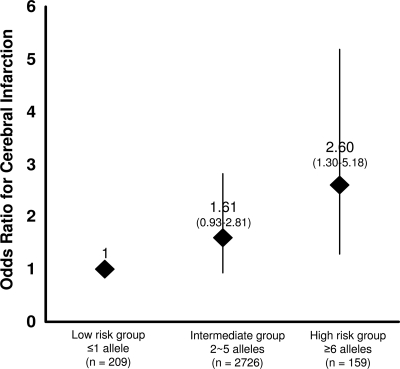
Next, the study subjects were divided into eight groups according to the total number of proatherothrombotic alleles they had. The prevalence of cerebral infarction in the subjects with zero (n = 19), one (n = 150), two (n = 525), three (n = 881), four (n = 826), five (n = 494), six (n = 138), and seven (n = 21) proatherothrombotic alleles were 5.3, 6.8, 9.5, 9.4, 11.5, 11.1, 15.2, and 19.0%, respectively. The proportion of patients with cerebral infarction increased progressively with increasing number of unfavorable alleles (P value for linear trend = 0.004) (Fig. 1). Furthermore, in a multiple logistic regression model, the number of proatherothrombotic alleles was significantly associated with cerebral infarction even after adjustment for sex, age, smoking habit, BMI, duration of diabetes, A1C, prevalence of hypertension, and prevalence of dyslipidemia (odds ratio [OR] for one-point increase in the number of proatherothrombotic allele 1.14 [95% CI 1.04–1.26], P = 0.006) (Table 2). The allele risk score was tested for its accuracy in the predicting the prevalence of cerebral infarction with receiver operating curve analysis. The area under the curve was 0.545 (95% CI 0.512–0.578) (P = 0.008). Using the median of the number of proatherothrombotic alleles as cutoff, patients with four or more alleles had a significantly increased risk of cerebral infarction as compared with subjects with three or less alleles (OR 1.38 [95% CI 1.08–1.76], P = 0.011, by multiple logistic regression). Using approximately the 5th and the 95th percentiles of the number of proatherothrombotic allele distribution (i.e., 1 and 6, respectively), the study population could be classified into three subgroups: carriers of one or less unfavorable alleles (n = 209), carriers of two to five unfavorable alleles (n = 2,726), and carriers of six or more unfavorable alleles (n = 159). The prevalence of cerebral infarction in these three groups increased progressively with the increase of unfavorable allele number (6.7, 10.4, and 15.7%, respectively, P value for trend = 0.006), while they were similar for the other clinical and laboratory variables (data not shown). Considering the carriers of one or less unfavorable allele as the reference group, the carriers of two to five alleles tended to have a relatively higher risk of cerebral infarction (1.61 [0.93–2.81], P = 0.092) and the carriers of six or more alleles had a significantly increased risk (2.60 [1.30–5.18], P = 0.007) (Fig. 2). The carriers of six or more alleles also had a significantly increased risk as compared with those with two to five alleles (1.61 [1.03–2.51], P = 0.036). Furthermore, even after adjusting other clinical variables, the carriers of six or more alleles had a significantly increased risk as compared with carriers of one or less allele (2.72 [1.30–5.69], P = 0.008), although there was no significant difference between the carriers of six or more alleles and the carriers of two to five alleles. These results suggest that the accumulation of proatherothrombotic alleles is associated with the prevalence of the cerebral infarction.
Figure 1.
The prevalence of cerebral infarction in the subjects with zero to seven proatherothrombotic alleles. Each number of proatherothrombotic alleles is shown as a vertical bar and the prevalence of cerebral infarction is shown as dots connected with lines. The prevalence of cerebral infarction tended to be greater as the number of proatherothrombotic alleles were increased. P for trend = 0.004.
Table 2.
Multivariate logistic regression analysis to identify independent determinants for cerebral infarction
| OR (95%CI) | P | |
|---|---|---|
| Sex (female) | 0.83 (0.59–1.17) | NS |
| Age (years) | 1.13 (1.11–1.15) | <0.001 |
| BMI (kg/m2) | 1.03 (0.99–1.07) | NS |
| Duration of diabetes (years) | 0.92 (0.90–0.95) | <0.001 |
| A1C (%) | 0.90 (0.81–1.02) | NS |
| Presence of hypertension | 2.53 (1.74–3.70) | <0.001 |
| Presence of dyslipidemia | 1.06 (0.79–1.43) | NS |
| Smoking (Brinkman's Index ≥200) | 1.67 (1.20–2.32) | 0.003 |
| Allele risk score | 1.14 (1.04–1.26) | 0.006 |
Multivariate logistic regression analysis was done for 3,094 type 2 diabetic patients to select variables significantly associated with an increase in the risk of cerebral infarction. The threshold of statistical significance was defined as P < 0.05. NS, not significant.
Figure 2.
OR for cerebral infarction in groups stratified on the basis of “unfavorable” proatherothrombotic alleles. Using approximately the 5th and the 95th percentiles of the number of proatherothrombotic allele distribution, the study population could be classified into three subgroups: carriers of one or fewer unfavorable alleles (n = 209), carriers of two to five unfavorable alleles (n = 2,726), and carriers of six or more unfavorable alleles (n = 159). Considering the carriers of one or fewer unfavorable allele as the reference group, the carriers of two to five alleles tended to have a relatively higher risk of cerebral infarction (OR 1.61 [95% CI 0.93–2.81], P = 0.092), and the carriers of six or more alleles had a significantly increased risk (2.60 [1.30–5.18], P = 0.007).
CONCLUSIONS
In the past decade, numerous polymorphisms have been reported as genetic risk factors for CVD. However, it remained controversial whether many of these polymorphisms are really associated with CVD because of their poor reproducibility. On the other hand, there is compelling evidence that CVD is a complex and multifactorial disorder and that its pathogenesis involves multiple molecules. These findings suggest that the effect of each genetic susceptibility factor for CVD is modest but might be important in the presence of other genetic risk factors. These gene-gene interactions might occur between different genes whose products are part of pathways that ultimately lead to CVD.
It is believed that disruption of vulnerable atherosclerotic plaque and subsequent thrombus formation play critical roles in the pathogenesis of cerebral infarction. In this study, we simultaneously determined four relatively common genetic variants related to plaque rupture and/or thrombus formation (MMP9 C-1562T, F12 C46T, VWF G-1051A, and PAI-1 −6754G/5G) and evaluated their combined effect on cerebral infarction in Japanese type 2 diabetic patients.
First, we performed the conventional single-association analysis, where the association between each polymorphism and the prevalence of cerebral infarction was examined individually. This analysis revealed that the prevalence of cerebral infarction tended to be higher with the increase of unfavorable allele number in each polymorphism. However, in any genetic models, there were no statistically significant associations between each polymorphism and the prevalence of cerebral infarction. Several previous case-control studies (6,15) showed that the MMP9 −1562T allele was associated with CAD, but the others, including a prospective study and a meta-analysis (7,16), failed to confirm the association. The association between cerebral infarction and this polymorphism has not been evaluated. Similarly, it is still controversial whether F12 C46T polymorphism is associated with CVD (11,17–19). A population-based case-control study in the Spanish population showed that C46 allele was a risk factor for cerebral infarction (18), but another study in the Japanese population failed to confirm the association (19). Regarding VWF G-1051A polymorphism, there have been few reports. Although a population-based case-control study in Caucasians indicated that this polymorphism was not associated with myocardial infarction (20), the association between this polymorphism and cerebral infarction has not been evaluated. Regarding PAI-1 −6754G/5G polymorphism, several population-based case-control studies have demonstrated an increased risk of CAD and cerebral infarction in carriers of 4G allele, but these findings have not been confirmed in larger studies (21). Although recent meta-analysis (22) indicated a weakly positive association of the 4G allele with the risk of CAD, the association between this polymorphism and cerebral infarction has not been demonstrated. Thus, the results of the previous studies were controversial regarding the association between CVD and each polymorphism, while most of them were population-based case-control studies and focused on CAD rather than cerebral infarction. Taking into account of these results and ours, it is likely that the effect of each polymorphism is not, at most, potent enough to definitively increase the risk of cerebral infarction in individuals with and without diabetes.
Next, we assessed the combined effect of these polymorphisms on cerebral infarction based on the working hypothesis that the accumulation of these polymorphisms could substantially influence the risk of atherothrombosis. We standardized the contribution of each genetic polymorphism and used the total number of proatherothrombotic alleles as an index of combined effect of these genes. Interestingly, there was a statistically significant graded relationship between the number of proatherothrombotic alleles and the prevalence of cerebral infarction (Fig. 1), and the number of proatherothrombotic alleles was an independent risk factor for the prevalence of cerebral infarction after adjusting other clinical variables (Table 2). These results suggest that the accumulation of proatherothrombotic alleles is associated with the prevalence of cerebral infarction independently of conventional risk factors.
Our study has several limitations. First, it is sometimes difficult to distinguish between cerebral hemorrhage and postinfarct cerebral hemorrhage when clinical course of the disease is unclear. Although the subjects who had apparent clinical course and/or the typical image findings of postinfarct cerebral hemorrhage were diagnosed as having cerebral infarction, we cannot exclude the possibility that several subjects who had postinfarct cerebral hemorrhage lesion were faultily counted as old cerebral hemorrhage but not as cerebral infarction.
Second, in order to avoid the type 1 error, which can be caused by multiple comparisons, we set a strict level of the statistical significance. Therefore, we cannot exclude the possibility that such strict level of the significance masked the weak association between each polymorphism and cerebral infarction.
Third, this study indicates that it is important to examine these polymorphisms together with predisposition to cerebral infarction in each individual. However, the true mechanisms of how the accumulation of these alleles influences the development of cerebral infarction should be evaluated in further studies. It is also noted that since the analysis was performed based on cross-sectional data, a long-term follow-up study will be required.
In addition, there was an unexpected inverse association between the duration of diabetes and the prevalence of cerebral infarction in this study. One possible explanation is that the duration of diabetes in some patients did not necessarily reflect the true duration of diabetes. Therefore, we performed another logistic regression analysis in which the duration of diabetes was excluded and confirmed that the number of proatherothrombotic alleles was still significantly associated with cerebral infarction (OR 1.15 [95% CI 1.05–1.26], P = 0.004) in this model (data not shown).
In conclusion, the accumulation of gene polymorphisms related to plaque rupture and thrombus formation is likely associated with the prevalence of cerebral infarction in type 2 diabetic patients.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain 2000; 123: 1784– 1812 [DOI] [PubMed] [Google Scholar]
- 2.Markus H. Genes for stroke. J Neurol Neurosurg Psychiatry 2004; 75: 1229– 1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions: association of active enzyme synthesis with unstable angina. Circulation 1995; 91: 2125– 2131 [DOI] [PubMed] [Google Scholar]
- 4.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apo E-deficient mice. J Clin Invest 2006; 116: 59– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantino Y, Nguyen TT, Wolk R, Aiello RJ, Terra SG, Fryburg DA. Potential imolications of matrix metalloproteinase-9 in assessment and treatment of coronary artery disease. Biomarkers 2009; 14: 118– 129 [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Ye S, Herrmann SM, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney AM. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation 1999; 99: 1788– 1794 [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret Lthe AtheroGene Investigators. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003; 107: 1579– 1585 [DOI] [PubMed] [Google Scholar]
- 8.Miller GJ, Esnouf MP, Burgess AI, Cooper JA, Mitchell JP. Risk of coronary heart disease and activation of factor XII in middle-aged men. Arterioscler Thromb Vasc Biol 1997; 17: 2103– 2106 [DOI] [PubMed] [Google Scholar]
- 9.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood 2008; 112: 3555– 3562 [DOI] [PubMed] [Google Scholar]
- 10.Kanaji T, Okamura T, Osaki K, Kuroiwa M, Shimoda K, Hamasaki N, Niho Y. A common genetic polymorphism (46 C to T substitution) in the 5-prime-untranslated region of the coagulation factor XII gene is associated with low translation efficiency and decrease in plasma factor XII level. Blood 1998; 91: 2010– 2014 [PubMed] [Google Scholar]
- 11.Kohler HP, Futers TS, Grant PJ. FXII (46C3T) polymorphism and in vivo generation of FXII activity-gene frequencies and relationship in patients with coronary artery disease. Thromb Haemos 1999; 81: 745– 747 [PubMed] [Google Scholar]
- 12.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation 2008; 117: 1449– 1459 [DOI] [PubMed] [Google Scholar]
- 13.Keightley AM, Lam YM, Brady JN, Cameron CL, Lillicrap D. Variation at the von Willebrand factor (vWF) gene locus is associated with plasma vWF:Ag levels: identification of three novel single nucleotide polymorphisms in the vWF gene promoter. Blood 1999; 93: 4277– 4283 [PubMed] [Google Scholar]
- 14.Dawson S, Wiman B, Hamsten A, Green F, Humphries S, Henney AM. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem 1993; 268: 10739– 10745 [PubMed] [Google Scholar]
- 15.Cho HJ, Chae IH, Park KW, Ju JR, Oh S, Lee MM, Park YB. Functional polymorphism in the promoter region of the gelatinase B gene in relation to coronary artery disease and restenosis after percutaneous coronary intervention. J Hum Genet 2002; 47: 88– 91 [DOI] [PubMed] [Google Scholar]
- 16.Abilleira S, Bevan S, Markus HS. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J Med Genet 2006; 43: 897– 901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endler G, Mannhalter C, Sunder- Plassmann H, Lalouschek W, Kapiotis S, Exner M, Jordanova N, Meier S, Kunze F, Wagner O, Huber K. Homozygosity for the C-T polymorphism at nucleotide 46 in the 5-prime untranslated region of the factor XII gene protects from development of acute coronary syndrome. Br J Haemat 2001; 115: 1007– 1009 [DOI] [PubMed] [Google Scholar]
- 18.Santamaría A, Mateo J, Tirado I, Oliver A, Belvís R, Martí-Fábregas J, Felices R, Soria JM, Souto JC, Fontcuberta J. Homozygosity of the T allele of the 46 C3T polymorphism in the F12 gene is a risk factor for ischemic stroke in the Spanish population. Stroke 2004; 35: 1795– 1799 [DOI] [PubMed] [Google Scholar]
- 19.Oguchi S, Ito D, Murata M, Yoshida T, Tanahashi N, Fukuuchi Y, Ikeda Y, Watanabe K. Genotype distribution of the 46 C/T polymorphism of coagulation factor XII in the Japanese population: absence of its association with ischemic cerebrovascular disease. Thromb Haemost 2000; 83: 178– 179 [PubMed] [Google Scholar]
- 20.Bitondo R, Cameron CL, Daly ME, Croft SA, Steeds RP, Channer KS, Samani NJ, Lillicrap D, Winship PR. The -1185 A/G and -1051 G/A dimorphisms in the von Willebrand factor gene promoter and risk of myocardial infarction. Br J Haematology 2001; 115: 701– 706 [DOI] [PubMed] [Google Scholar]
- 21.Simmonds RE, Hermida J, Rezende SM, Lane DA. Haemostatic genetic risk factors in arterial thrombosis. Thromb Haemost 2001; 86: 374– 385 [PubMed] [Google Scholar]
- 22.Ye Z, Liu EH, Higgins JP, Keavney BD, Lowe GD, Collins R, Danesh J. Seven haemostatic gene polymorphisms in coronary disease: meta-analysis of 66,155 cases and 91,307 controls. Lancet 2006; 367: 651– 658 [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med 2002; 347: 1916– 1923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.