Operationally, up and down states refer to the observation that neurons have two preferred subthreshold membrane potentials, both subthreshold for action potential generation. The most common method of detecting these states is the so-called all-points histogram (Cowan and Wilson, 1994; Wilson and Kawaguchi, 1996; Paré et al., 1998). This method, borrowed from the study of single channel currents, plots the frequency of occurrence of various values of membrane potential for every point in a digitized record. Figure 1 shows a typical example for a striatal spiny neuron, and for a cortical pyramidal cell in layer V, recorded simultaneously. The histogram to the left of each one shows the amount of time the cell spends at each value of membrane potential. Both cells toggle between two preferred membrane potentials, one very hyperpolarized (Down state), and one more depolarized (Up state). In both cells, the Up state is only a few millivolts from the action potential threshold. Usually, membrane potential fluctuations around the Up state are of higher amplitude, whereas the Down state is relatively free of noise.
Figure 1.
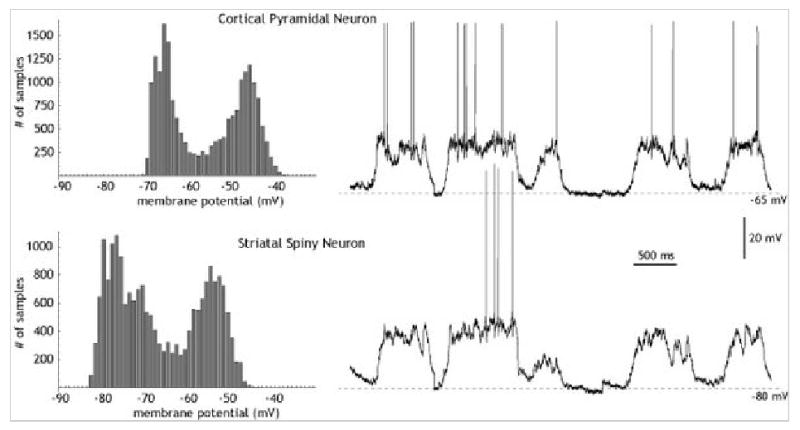
Up and down states.
Up and Down states have been most often studied in animals anesthetized with urethane or other anesthetics that induce slow coherent oscillations in the cortex similar to those seen during sleep or anesthesia (e.g. Steriade et al., 1993; Steriade et al., 2001; Mahon et al., 2006; Destexhe et al. 2007). Because of this, Up and Down states are sometimes used as a synonym for slow oscillations. That usage is avoided here, and, Up and Down states will refer only to the set of cellular and network properties that causes neurons to respond to synaptic input in a two-state manner. These cellular properties arise from ionic conductances that are always present in the cell, and can continue to influence cellular activity in other circumstances not associated with slow oscillations. For example, much of the work on Up and Down states has been obtained in tissue slices, which are neither anethetized nor asleep, and often not showing any slow oscillations.
Neurons may exhibit two-state behavior because of their intrinsic properties, or because they are in a network that imposes it on them, or both, and may be expressed as a part of a variety of activity states.
Plateau potentials and cellular bistability
The mechanisms that give rise to preferred membrane potentials can best be categorized according to the intrinsic stability of each state. In both the cerebral cortex and the striatum, the Down state for individual neurons is stable, and is close to the resting membrane potential observed when all input is removed (Wilson et al., 1983b; Timofeev et al., 2001). This does not mean that in those cells the Up state may not also be stable. It is well known that some neurons are bistable, meaning that they simultaneously possess stable depolarized, as well as hyperpolarized states and once placed in either state will remain there with no additional stimululation. The stable depolarized states exhibited by some motoneurons and by cerebellar Purkinje cells are good examples (Hounsgaard and Kiehn, 1985;; Loewenstein et al., 2005). Many other neurons show metastable, or transiently stable depolarized states usually called plateau potentials, which can be entered by brief depolarizations and which last for some period of time before the membrane potential spontaneously returns to its resting state (Llinás and Sugimori, 1980). In these cases, stability of the membrane potential during the plateau is lost gradually due to some slow process caused by depolarization gradually accumulates and destabilizes the plateau.
An appropriate test for stability of the Up state is experimental depolarization of the membrane by current injection at a time when the cell would otherwise be in the Down state. When the current is released, a bistable neuron would remain in the Up state until perturbed again. Lingering in the Up state, followed by a return to the Down state after a significant delay, would be indicative of the operation of a transiently stable Up state. Likewise, attempts have been made to evoke a stable transition to the Down state by briefly hyperpolarizing neurons to the potential associated with the Down state. An Up state stabilized by regenerative voltage dependent electrical activity should be vulnerable to termination in this way.
Bistable networks and balanced inhibition
Bistable networks may produce two-state behavior in cells possessing no intrinsic bistability. An early description of this kind of network was presented by Wilson and Cowan (1972) as an abstract model of cortical circuits. This simple model consisting of a population of excitatory neurons and a population of inhibitory neurons mutually connected and each connected among themselves. Depending on the strengths of synaptic connections, the model could produce a variety of different patterns of activity, including stability of low and high activity states. The two states were divided by a threshold, but to toggle the network between states it was not sufficient to change the activity of one cell but required a transient change in a substantial number of cells. The stable level of activity in both the excited and quiet states of the Wilson and Cowan model is determined by a balance between the mutual excitation among excitatory neurons and the feedback inhibition they generate by the way of the inhibitory population. Most network models of Up and Down states in the cortex are extensions of the Wilson and Cowan model. They employ mutual excitation among cortical pyramidal cells to achieve a threshold level of excitation beyond which the population can regeneratively self-excite. Creation of a stable Up state at moderate membrane potentials is achieved by balancing excitation with inhibition from interneurons.
It should be noted that (like the Wilson and Cowan model) this mechanism can readily be altered to generate rhythmic transitions between excited and quiet states of the network. To achieve this, the Up and Down states must both be made to be transiently stable. Current biophysically realistic versions of the network model employ synaptic depression or accumulation of afterhyperpolarization currents to destabilize it with time (Bazhenov et al., 2002; Compte et al., 2003;Holcman and Tsodyks, 2006; Yuste et al., 2005). Periodic re-entry into the Up state is achieved by postulating a stochastic mechanism that randomly triggers a transition to the excited network state from time to time.
Striatal spiny neurons
Up and Down states were first described in striatal spiny neurons (Wilson and Groves, 1981). These neurons receive thousands of excitatory synaptic inputs located on their dendritic spines, but the spiny cells are inhibitory cells, and the striatal network lacks a source of excitatory feedback. The occurrence of Up and Down states in striatal neurons required phasic changes in the cortical and thalamic inputs to striatal neurons (O'Donnell and Grace, 1995; Wilson, 1986; Wilson et al., 1983a; Wilson et al., 1983b; Wilson and Kawaguchi, 1996; Kasanetz et al., 2006). Much of this work was done using in vivo recording in urethane anesthetized animals, in which the activity of cortical, thalamic and striatal neurons is dominated by slow (∼ 1Hz) oscillations (Contreras et al., 1995). It also made use of the ability of cortical and thalamic stimulation to reset the slow oscillation. Acute cortical lesions abolished the slow oscillations seen in the striatum, although stimulation of the remaining axons of the ablated cells could still evoke monosynaptic responses in the striatal cells. Cortical and thalamic stimulation also reset the slow oscillations of cortical neurons specifically projecting to the striatum, and so produced periods of silence and enhanced activity that precisely corresponded to the Down and Up states seen in striatal cells. When cortical (and thalamic) inputs were silenced by lesions or by stimulation, all striatal spiny cells exhibited stable membrane potentials associated with the Down state. Striatal cells recorded in brain slices that interrupt the projections from the cerebral cortex always show only one stable membrane potential, associated with the Down state. During slow oscillations in vivo, Up and Down state in widespread areas of the cerebral cortex are nearly synchronous (Volgushev et al., 2006), and striatal spiny cells located as far as millimeters away likewise enter and leave the Up state within a few milliseconds of each other (Stern et al., 1998).
Ionic mechanism of the down state in striatal spiny neurons
The stabiliity of the Down state is apparent from examination of the current-voltage (I-V) curve for the spiny neuron. At membrane potentials associated with the Down state, the I-V curve slope (the input conductance) increases with hyperpolarization. This is called inward rectification, or anomalous rectification (anomalous because it isn't predicted by the Goldman-Hodgkin-Katz equation). In the Down state, the input resistance of the spiny neuron is low (10-30 MOhms), making the cell relatively insensitive to small synaptic inputs. The origin of inward rectification in the spiny neuron is a hyperpolarization-activated potassium channel, KIR2 (Nisenbaum and Wilson, 1995). The KIR2 channel is regenerative in the sense that it is a hyperpolarizing channel that is activated by hyperpolarization. In the Down state, when synaptic input is low, the conductance contributed by this channel is much larger than any other influence on the spiny neuron. The spiny cell's membrane potential moves close to the potassium reversal potential, and the cell enters a high-conductance state, in which it is relatively insensitive to synaptic input. Because the membrane potential in the Down state is more hyperpolarized than the chloride reversal potential, GABAA-mediated inhibitory synapses from striatal interneurons and from recurrent collaterals of other spiny neurons are all depolarizing in nature (Plenz, 2003).
Ionic mechanism of the up state in striatal spiny neurons
At depolarized potentials associated with the Up state, the I-V curve shows a strong curvature in the other direction (outward rectification). Input resistance in this voltage range state decreases with depolarization because of the activation of depolarization-activated K+ channels. The spiny striatal neuron possesses several of these that are activated in the range associated with the Up state, but with a prominent contribution from channels in the Kv1 family (Shen, et al. 2004). Unlike the KIR2 current, the currents through depolarization-activated potassium channels are not regenerative, but rather restorative, as they tend to return the membrane potential to the Down state. In the presence of strong depolarizing current from excitatory synaptic input, they act to limit the sensitivity of the neuron to those inputs. When the membrane potential has achieved a level at which these channels are strongly activating with additional depolarization, any additional depolarization that is achieved by synaptic input must provide enough current to counteract the voltage-dependent increase in K+ channel activation that results from that depolarization. Outwardly-rectifying K+ channels in spiny neurons are in the steep part of their activation range in the Up state (Nisenbaum et al. 1996), so very large changes in synaptic input are required to achieve even small increases in depolarization, and the membrane potential remains relatively constant despite slow fluctuations in the level of synaptic input. Voltage-activated potassium channels do not activate and deactivate rapidly like the KIR2 channels responsible for the Down state. Rapidly changing synaptic currents may be able to produce brief depolarizations the opportunity to depolarize the cell before voltage sensitive channels can be recruited to oppose them (Wilson, 1995). This makes the striatal spiny cell sensitive to brief and sudden changes in the level of excitation in the Up state, and these can trigger action potentials in an irregular pattern. Spiny neurons usually do not fire rhythmically in response to constant depolarizing current from the Up state, although they do respond in this way to constant current applied in the Down state.
Are striatal spiny neurons bistable?
Between the Down and Up states there is a region of membrane potential in which neither set of K+ channels are strongly activated. In this range, the input resistance of the spiny neuron is much greater, the time constant is much longer, and small synaptic inputs are more effective. Moreover, in part of this voltage range, KIR2 currents deactivate regeneratively. In the transition between the Up and Down states, any regenerative current could readily create a forbidden region of membrane potentials, some range in which if the potential reached that point it would be regeneratively moved either in the depolarizing or hyperpolarizing direction. Persistent sodium currents, and voltage-dependent calcium currents, have both been demonstrated to be present in the spiny neuron, and to be activated to some degree in the voltage ranges required. The voltage sensitivity of the NMDA channel offers an especially interesting possibility, because it combines the slow dynamics of glutamate binding at the NMDA receptor with the rapid voltage sensitive block and unblock of the NMDA receptor channel (Wolf et al.,2005). In all of these cases, it is possible to show in simulations how these mechanisms could generate bistability of the striatal spiny neuron (Gruber et al. 2003). For the limited range of in vivo preparations that have been studied so far, there has been no evidence of discontinuities in the I-V relationship, bistability, or forbidden values of the membrane potential. Depolarizing current pulses presented in the Down state do not generate Up states in striatal spiny neurons, and it is likewise impossible to terminate an Up state by application of any current transient applied to the cells. During slow oscillations in the cortex, the onset and offset of Up and Down states in individual spiny neurons in the striatum are highly synchronized, offering no indication of modification by striatal neurons of the timing of these transitions (Stern et al., 1998). For the striatal neuron in vivo for all preparations studied so far, only the Down state is stable. The nonlinear properties of the striatal neuron sharpen up the temporal onset and offset of the envelope of synaptic input, and buffer the membrane potential to reduce its variability in the hyperpolarized and depolarized range, but do so in a soft manner.
Does balanced inhibition contribute to the up state in striatal spiny neurons?
Excitatory input to the striatum generates local inhibition via feedforward and feedback pathways. Feedforward inhibition is generated by inhibitory GABAergic interneurons that receive excitatory inputs from the same sources as the striatal spiny neurons. Feedback inhibition is generated by the recurrent axon collaterals of the spiny neurons themselves. A direct measure of the contribution of inhibition to the synaptic input to the spiny neuron in the Up state can be obtained by measurement of the reversal potential of the Up and Down states after removal of voltage-sensitive conductances by intracellular application of blockers (Wilson and Kawaguchi, 1996). The conductance in the Up state under these circumstances is much greater than the Down state, reflecting the contribution of synaptic input. For the striatal cell Up state, the reversal potential was -10 to -20 mV. For excitation and inhibition to be in balance, the Up state membrane potential would have to be near this value. The synaptic potential evoked by cortical stimulation reversed at the same membrane potential as the Up state, consistent with the Up state being essentially a maintained excitatory input barrage to the striatal spiny neuron, balanced primarily by voltage-sensitive potassium currents.
Cortical pyramidal cells
Like striatal spiny cells, many cortical pyramidal cells also toggle between preferred depolarized and hyperpolarized subthreshold membrane potentials, and spontaneous switching between these membrane potential states is most conspicuous when the cortex is exhibiting synchronized slow wave oscillations (Cowan and Wilson, 1994; Timofeev et al., 1996). Strong electrical stimuli applied to the cortex or thalamus resets the slow oscillation, resulting in a reliable Down state followed reliably by an Up state. Slow oscillations can also be observed in isolated slabs and slices, and slice cultures of cerebral cortex under appropriate circumstances (Sanches-Vives and McCormick, 2000; Timofeev et al., 2000; Plenz and Aertsen, 1996), and even in isolated slices of the thalamus (Hughes et al., 2002). These phenomena offer the opportunity for a clear dissection of the ionic currents responsible for the Up and Down states, within the context of the slow oscillation. Because the slow oscillation is largely generated locally within the cortex, Up and Down states in cortical pyramidal cells are not as easily separated from slow oscillations as they are in the striatum.
The down state of the cortical pyramidal neuron
Although inward rectification is a common feature of cortical pyramidal neurons, they generally do not show as strong inward rectification as striatal spiny cells, and there is evidence that inwardly rectifying K+ currents other than KIR2 may be critical, at least in some kinds of pyramidal cells (Cunningham et al., 2006). Despite this difference, the fundamental properties of Down states in pyramidal neurons are roughly the same as those of the striatal spiny cell. That is, the Down state is a stable equilibrium achieved during periods of decreased synaptic input. Intracellular infusion of unspecific K+ channel blocker, Cs+, abolishes hyperpolarization associated with down states during sleep (Timofeev et al., 2001). Hyperpolarization produced by reduction of synaptic excitation, rather than to an increase in inhibition, is called disfacilitation, and this has been shown to be the mechanism responsible for spontaneous Down states during slow oscillations of the cortex and in the Down state triggered by cortical or thalamic stimulation (Shu et al., 2003; Sanchez-Vives and McCormick, 2000; Waters and Helmchen, 2006; Timofeev et al., 2000; Contreras et al., 1996). Most excitatory input to cortical pyramidal cells arises from thalamocortical neurons or other pyramidal cells, so the Down state must arise from some influence that quiets these inputs.
The up state in the cortex
Cortical pyramidal cells have a variety of somato-dendritic regenerative currents that could produce stability or metastability in the Up state, but physiological studies have not revealed widespread bistability among cortical pyramidal cells in vivo. Here there is a place for caution because of the heterogeneity of cortical pyramidal cell types. For example, some cortical pyramidal cells, called intrinsic bursting cells, regenerative cellular activity is much more evident than in other pyramidal cells, which are usually considered to be regular spiking (Connors and Gutnick, 1990), and these cell may have a special role in cortical dynamics (Timofeev et al., 2000). Still, most cortical pyramidal cells that exhibit clear Up and Down states do not show any indication of cellular stability in the Up state. The somato-dendritic membrane nonlinearity responsible for the Up state in striatal cells have been observed in cortical pyramidal cells (Waters and Helmchen, 2006), and NMDA-dependent plateau potentials have been proposed as a potential mechanism (Milojkovic et al. 2005), but most attempts to explain the Up and Down state have featured network, rather than cellular properties to explain the Up state.
Inhibition is everywhere apparent in the cortex. Any strong stimulus applied to the cortex evokes a clear IPSP component as a part of the response. Both feedforward and feedback inhibition in the cortex are mediated by the wide variety of GABAergic interneurons, and any activation of pyramidal neurons invariably leads to a graded inhibitory response that could act to balance any mutual excitatory effects among pyramidal cells (Haidner et al., 2006; Rigas and Castro-Alamancos, 2007; Destexhe et al. 2003; Rudolph et al., 2007). This offers a simple network mechanism that could create Up and Down states in pyramidal cells and not require any particular cellular mechanisms like those in striatal cells, and that mechanism has been studied in a number of variants (Bazhenov et al., 2002; Compte et al., 2003; Holcman and Tsodyks, 2006; Yuste et al., 2005). In all, the Down state of the network is a state of mutually-enforced quiet. Any input to any subset of cells will trigger some mutual excitation and some associated inhibition. If enough excitation is present, the network will re-excite itself explosively, and the cells will depolarize toward the Up states. The inhibition generated by pyramidal cell activity will also be recruited,, and the combined excitatory and inhibitory conductance will impose a more negative reversal potential for the net conductance change. If the balance of excitation remains high enough to maintain self-sustained activity, the effect of the network on any one cell will appear as a synaptic conductance with the reversal potential set by the balance of excitation and inhibition. If this balanced membrane potential were reliably subthreshold, the network could not maintain self-sustained activity and the Up state would fail. But because random (or oscillatory) fluctuations in membrane potential in the Up state trigger some action potentials in at least some of the neurons, the Up state may sustain itself as a stable or at least a transiently stable state. Some accumulating fatigue, due perhaps to short term synaptic plasticity, AHP currents or other similar mechanism, makes the Up state gradually lose stability, returning the network to the Down state. After some time in the Down state to recover, the network would be ready for another Up state transition, but would need some additional stochastic mechanism that would produce the next Up state. The thalamic input is an obvious choice for this mechanism, but it has been shown that slow oscillations (although slower than that seen in vivo) can be seen in the absence of thalamic input to the cortex. Thus although the thalamus may regulate timing of slow oscillations in vivo, the cortex can create Up states without that input (see review in Destexhe and Sejnowski, 2003).
Figure 2.
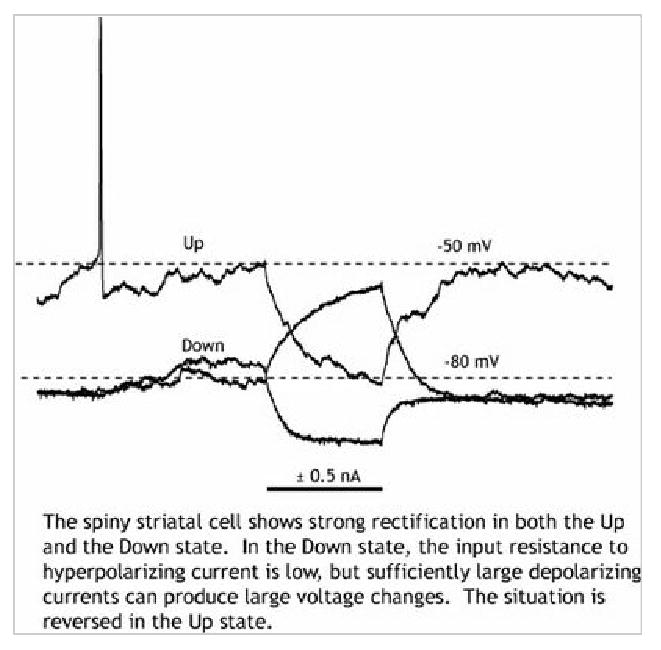
Measurement of input resistance and rectification with a small depolarizing or hyperpolarizing pulse in the Up and Down states
Figure 3.
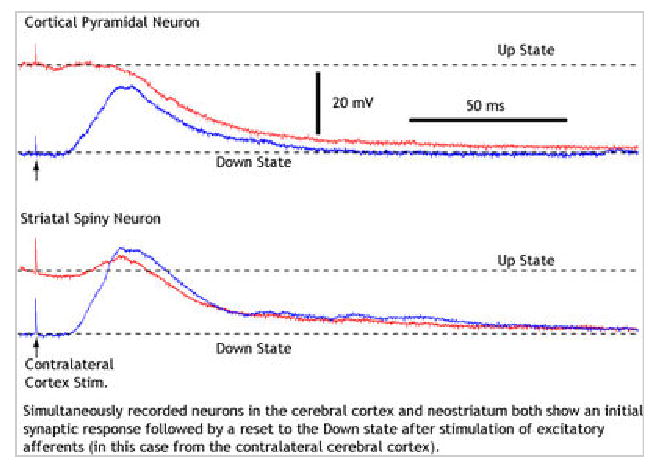
EPSPs and Down state transitions evoked in cortical and striatal neurons by cortical stimulation
References
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. TINS. 1990;1:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15 doi: 10.1523/JNEUROSCI.15-01-00604.1995. 6040622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RSG, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. PNAS. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? TINS. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. the high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev. 2003;83:1401–1453. doi: 10.1152/physrev.00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Solla SA, Surmeier DJ, Houk JC. Modulation of striatal single units by expected reward: a spiny neuron model displaying dopamine-induced bistability. J Neurophysiol. 2003;90:1095–1114. doi: 10.1152/jn.00618.2002. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Tsodyks M. The emergence of up and down states in cortical networks. PLOS Computational Biology. 2006;2:174–181. doi: 10.1371/journal.pcbi.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, O'Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. J Physiol. 2006;577:97–113. doi: 10.1113/jphysiol.2006.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Häusser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci. 2005;8:2002–211. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3940–3951. doi: 10.1523/JNEUROSCI.5314-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ, Foehring RC, Surmeier DJ. Isolation and Characterization of a Persistent Potassium Current in Neostriatal Neurons. J Neurophysiol. 1996;76:1180–1194. doi: 10.1152/jn.1996.76.2.1180. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H, Destexhe A, Lang E. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interactions between spiny projection neurons in striatal function. TINS. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Plenz D, Aertsen A. Neural dynamics in cortex-striatum co-cultures--II. Spatiotemporal characteristics of neuronal activity. Neuroscience. 1996;70:893–924. doi: 10.1016/0306-4522(95)00405-x. [DOI] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical up states: Differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;16:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shen W, Hernandez-Lopes S, Tkatch T, Held JE, Surmeier DJ. KV1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons. J Neurophysiol. 2004;91:1337–1349. doi: 10.1152/jn.00414.2003. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at diffferent levels of vigilance. Cerebral Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:2352–2365. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Contreras D, Steriade M. Synaptic responsiveness of cortical and thalamic neurons during various phases of slow sleep oscillation in cat. J Physiol. 1996;494:265–278. doi: 10.1113/jphysiol.1996.sp021489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. PNAS. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Murovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations. J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Background synaptic activity is sparse in neocortex. J Neurosci. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Postsynaptic potentials evoked in spiny neostriatal projection neurons by stimulation of ipsilateral or contralateral neocortex. Brain Res. 1986;367:201–213. doi: 10.1016/0006-8993(86)91593-3. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Dynamic modification of dendritic cable properties and synaptic transmission by voltage-gated potassium channels. J Computational Neurosci. 1995;2:91–115. doi: 10.1007/BF00961882. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Origins of postsynaptic potentials evoked in spiny neostriatal projection neurons by thalamic stimulation in the rat. Exp Brain Res. 1983a;51:217–226. doi: 10.1007/BF00237197. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Disfacilitation and long-lasting inhibition of neostriatal neurons in the rat. Exp Brain Res. 1983b;51:227–2. doi: 10.1007/BF00237198. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O'Donnell P, Finkel LF. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, MacLean JN, Smith J, Lasner A. The cortex as a central pattern generator. Nature Reviews Neuroscience. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
Internal references
- Redgrave Peter. Basal ganglia. Scholarpedia. 2007;2(6):1825. [Google Scholar]
- Braitenberg Valentino. Brain. Scholarpedia. 2007;2(11):2918. [Google Scholar]
- Izhikevich Eugene M. Bursting. Scholarpedia. 2006;1(3):1300. [Google Scholar]
- Meiss James. Dynamical systems. Scholarpedia. 2007;2(2):1629. [Google Scholar]
- Izhikevich Eugene M. Equilibrium. Scholarpedia. 2007;2(10):2014. [Google Scholar]
- Roberts Eugene. Gamma-aminobutyric acid. Scholarpedia. 2007;2(10):3356. [Google Scholar]
- Destexhe Alain. High-conductance state. Scholarpedia. 2007;2(11):1341. [Google Scholar]
- Jonas Peter, Buzsaki Gyorgy. Neural inhibition. Scholarpedia. 2007;2(9):3286. [Google Scholar]
- Moehlis Jeff, Josic Kresimir, Shea-Brown Eric T. Periodic orbit. Scholarpedia. 2006;1(7):1358. [Google Scholar]
- Holmes Philip, Shea-Brown Eric T. Stability. Scholarpedia. 2006;1(10):1838. [Google Scholar]
- Pikovsky Arkady, Rosenblum Michael. Synchronization. Scholarpedia. 2007;2(12):1459. [Google Scholar]
- Sherman S Murray. Thalamus. Scholarpedia. 2006;1(9):1583. [Google Scholar]


