Abstract
Abstract Genetic manipulation of embryonic stem (ES) cells has been used to produce genetically engineered mice modeling human disorders. Here we describe a novel, additional application: selection for a phenotype of interest and subsequent transmission of that phenotype to a living mouse. We show, for the first time, that a cellular phenotype induced by ENU mutagenesis in ES cells can be transmitted and recapitulated in adult mice derived from these cells. We selected for paraquat-resistant (PQR) ES clones. Subsequent injection of these cells into blastocysts resulted in the production of germline chimeras, from which tail skin fibroblasts exhibited enhanced PQR. This trait was also recovered in progeny of the chimera. We avoided PQ toxicity, which blocks the ability to involve the germline, by developing a sib-selection method, one that could be widely applied wherever the selection itself might diminish the pluripotency of the ES cells. Thus, phenotype-driven screens in ES cells are both feasible and efficient in producing intact mouse models for in vivo studies.
Introduction
Phenotype-driven screens of mutagenized populations of cells underlie much of the advances made in modern genetic research in the last century (Botstein and Maurer 1982). Such endeavors, however, were limited mostly to lower organisms. Although regional or genome-wide mutagenesis and phenotypic screens have been carried out in mice by several large laboratories, the operational burden in cost and time was extremely high. Large-scale mutagenesis in mouse embryonic stem (ES) cells not only offers a viable shortcut in generation of novel mouse mutants, it also provides possibilities not available in traditional in vivo studies of mice (Chen et al. 2000; Munroe et al. 2000). One such possibility is to screen for mutant phenotypes directly in ES cells and then in subsequent analyses to explore molecular genetic correlates of these phenotypic alterations. Unfortunately, current technology limits exploration of ES cell phenotypes to analyses at the level of the individual cell (Guo et al. 2004; Yusa et al. 2004). Here we exploit the pluripotency of ES cells to generate living mice that express the mutant phenotype observed in the ES cells. We have concentrated on identifying mutants that are resistant to oxidative stresses, a trait frequently associated with life extension in a variety of invertebrate species (Lin et al. 1998; Lithgow and Walker 2002) and also in the mouse (Holzenberger et al. 2003; Murakami et al. 2003). We and many others have exploited this oxidant-resistance property as a rapid means of identifying novel mutants in invertebrates that have the potential to extend longevity and reduce rates of aging (de Castro et al. 2004; Martin 2005). Here we report success in screening for an oxidant-resistance phenotype in ES cells and subsequent transmission to intact mice. Using standard blastocyst injections, we generated mice that were resistant to oxidative stress at the cellular level; this resistance phenotype was transmitted to the next generation.
Materials and methods
ES cell culture
We used a F1 hybrid (C57BL/6 J × 129X1/SvJ) ES cell line (c7.1) that was shown to maintain germline competency after extensive in vitro manipulation (Chick et al. 2005). c7.1 ES cells were cultured in ES cell medium [DMEM with high glucose, 15% fetal bovine serum, 1000 U/ml leukemia inhibitory factors (ESGRO, Chemicon), 0.1 mM nonessential amino acids, 2 mM GlutaMAX, 55 μM β-mercaptoethanol, and 25 U/ml penicillin/streptomycin] on a layer of mitomycin C-treated primary murine embryonic fibroblasts [PMEF, FVB-Tg(PGK-neo)]. We typically passage the ES cells every other day in a ratio of 1:7 to 1:10.
ENU mutagenesis and mutation rate estimation
ES cells (c7.1) were trypsinized with 0.25% trypsin-EDTA into a single-cell suspension. After washing to deplete trypsin, we incubated 3 × 106 ES cells in 6 ml of ES cell medium containing 0.5 mg/ml of ENU, with gentle rocking at 37°C for 2 h. Cells were then washed with PBS and diluted with ES cell medium to 1500 cells/ml and 100-μl aliquots were seeded into each well of a 96-well plate containing a monolayer of PMEF as feeder cells. A total of ten 96-well plates of mutagenized ES cells were prepared. About 90% of the ES cells were killed by the ENU treatment. On average, we observed six ES cell colonies per well. Thus, in a ten-plate set we had about 6000 clones surviving mutagenesis. These ES cells were then screened for PQ resistance.
To estimate the mutation frequency, we treated HSV-Tk-targeted c7.1 ES cells (clone 2C1) (Chick et al. 2005) with vehicle alone, 0.3 mg/ml, or 0.5 mg/ml of ENU for 2 h at 37°C. Treated ES cells were plated onto 100-mm culture dishes (with PMEF as feeder cells). After 2 days, ES cells were treated with FIAU (2′-deoxy-2′-fluoro-β-d-arabinofuranosyl-5-iodouracil) for 4 days to select against cells expressing functional thymidine kinase (Tk). We then counted the number of surviving colonies, presumably conferred by loss-of-function mutational events at Tk, as a measure of ENU mutagenicity.
Sib-selection for PQR ES cell clones
The ten-plate set of mutagenized ES cells was allowed to grow for 2 days after which one-fifth of the cells were replicated to another ten-plate set (gelatin-coated without feeder cells). The rest were frozen as master plates in ES cell medium with 20% FBS and 10% DMSO. We applied PQ (40 μM) in modified ES cell medium (5% FBS, without β-mercaptoethanol) to the screening plates of ES cells. The combination of low cell density, reduced FBS content, and withdrawal of β-mercaptoethanol in the media is critical in identification and recovery of PQR ES cells. After 2 days of PQ treatment, ES cells were cultured in ES cell medium until surviving colonies could be seen, typically 5-7 days later. Culture wells containing surviving ES cells were identified. Since each master well, on average, contained six clones of ES cells, a subcloning step was necessary to isolate the clonal-resistant ES cells. ES cells from the corresponding wells of the master plates were thawed and subcloned by seeding 1000 ES cells onto a 100-mm culture dish so that well-spaced individual ES cell colonies would form. We hand-picked 48 ES cell colonies onto a 96-well plate, one colony per well (48 wells per isolate). Each ES cell colony was trypsinized and replated and used for another round of sib-selection to identify clones of PQR cells. The PQR ES clone in the second master plate was expanded to establish a PQR ES cell line (now clonal).
Stress resistance test of ES cells
ES cells were treated with PQ to test for resistance to the oxidative stressor. We seeded 5000 cells onto a 60-mm gelatinized culture plate, in triplicate. Each replicate was treated with 5, 10, 15, 20, and 30 μM PQ in modified ES cell medium; an untreated triplicate served as a control. After 7 days of treatment the number of surviving ES cell colonies was counted.
Reactive oxygen species (ROS) production
The level of ROS production was measured using 2′, 7′-dichlorodihydrofluorescein diacetate (D399, H2DCFDA, Molecular Probes, Carlsbad, CA), as described (Perla et al. 2008). ES cells (30,000) were cultured on gelatin-coated 48-well plates at 37°C for 24 h. After removing the culture medium, cells were loaded with 10 μM H2DCFDA in PBS and incubated for 30 min at 37°C. PQ was then added from 100 μ stocks to the appropriate wells. Total ROS production was measured by a fluorescent plate reader at 485/538 nm after a 6-h treatment of stressor. The fluorescent signal obtained from untreated cells was designated as the basal ROS level to which treated cells were compared and calculated for the percentage increase in ROS. The increases in ROS production in response to paraquat between the parental wild-type cells and stress-resistant cells were analyzed by one-way ANOVA.
Generation of mice
Resistant ES cells were injected into C57BL/6 blastocysts, which were then implanted into a pseudopregnant ICR female. Chimera produced were mated to C57BL/6 females to test for germline transmission and to produce F1 progeny.
Stress resistance test on tail skin-derived fibroblasts
We isolated and processed tail biopsies from mice as described (Salmon et al. 2005). Tail skin fibroblasts isolated from the chimera and its F1 progeny were tested for PQ resistance; C57BL/6 × 129X1/SvJ hybrids served as a wild-type control (same mixed genetic background as the experimental mice). We seeded fibroblasts (at passage 3) onto a 96-well plate, 15,000 cells per well (in triplicate), and allowed for attachment in 24 h of culture. The culture medium was then aspirated and PQ was applied to treat the cells for 6 h. After that, normal culture medium was reinstated for recovery. After 18 h, viable cells surviving the stressor treatment were measured by a WST-1 cell proliferation assay. Cell viability was expressed as absorbance at 450 nm. Control wells containing untreated fibroblasts were used to normalize to 100% survival. The percentage of survival measured at 2 mM PQ was used for statistical analysis. Differences between experimental mice and wild-type control were evaluated by one-way ANOVA.
Results
ENU mutagenesis in ES cells
We used ENU (N-ethyl-N-nitrosourea) to mutagenize ES cells because of its generality and high efficiency, which allows easy saturation of the genome with newly induced mutations. ENU has been widely used in generating allelic series of mutations, including null, hypomorphic, and hypermorphic alleles (Justice et al. 1999), a feature desirable for encompassing a broad spectrum of mutations in a given screening pool. We estimated the efficiency of ENU mutagenesis in c7.1 ES cells (a hybrid of C57BL/6 and 129X1/SvJ) using an HSV-Tk-targeted c7.1 ES cell line (Chick et al. 2005). We were able to achieve a loss-of-function mutation rate of 0.1% at the targeted Tk gene with 0.5 mg/ml of ENU (Table 1). At this ENU concentration we generated an ES cell library of 6000 mutagenized clones, distributed in ten 96-well plates at about six clones per well. Considering the mouse genome has about 23,435 known protein-coding genes (Ensembl 2007), each mutagenized ES cell would have about 23 mutations (assuming the same target size as Tk). Thus, this ES cell library represents sixfold genomic coverage (Table 2).
Table 1.
ENU-induced mutation frequency (HSV-tk transgene) in ES cells
| ENU concentration |
No. of clones screened |
No. of FIAU- resistant clones |
Loss-of-function mutation frequency |
|---|---|---|---|
| 0.3 mg/ml | 15,200 | 7 | 0.46 × 10−3 |
| 0.5 mg/ml | 9,120 | 9 | 0.99 × 10−3 |
ES cells were selected for FIAU resistance 2 days post ENU treatment. The total number of colonies screened was estimated by plating ES cells on a separate plate not treated with FIAU
Table 2.
Summary of ENU mutagenesis and PQ selection of c7.1 ES cells
| Total No. of ENU-treated ES cells plated | 1.5 × 105 |
| No. of ENU-treated ES cells seeded per well (96-well plate) | 150 |
| No. of ES cell colonies growing per well | 6 (average) |
| Total no. of ES cell colonies screened | 6000 |
| Genomic coverage | Sixfold |
| No. of wells exhibiting PQ resistance | 7 |
| No. of PQ-resistant ES cell lines established | 6 |
Stress selection of ES cell clones by replica plating
We exposed this library of mutagenized ES cells to paraquat (PQ), a reactive oxygen species (ROS) generator, to select for PQ-resistant (PQR) mutants. As previously reported (Martin 2005), ES cells subjected to oxidative stress (95% oxygen) had very low efficiencies of chimerization after injection into blastocysts, presumably because exposure to the oxidative stress led to chromosomal lesions and the loss of pluripotency. To circumvent this problem, we devised a sib-selection methodology using replica plating (Fig. 1). From a fresh library containing 6000 independent ES cell mutant clones not exposed to stress, we replicated one-fifth of the cells onto another set of ten plates and screened them for PQR; the originals were frozen as a master set. Surviving ES cells were found in seven wells, following the PQ selection. We identified the corresponding wells in the master set and thawed ES cells from the frozen master stock for further work. Since each well of the master stock contains, on average, six independent ES clones, a subcloning step was necessary to isolate clonal PQR ES cells. Due to the low frequency of PQR mutants, it is likely that only one clone in any well was PQR. After subcloning we were able to isolate PQR ES cells from six of the seven wells (Table 2). One well of PQR cells did not grow well in culture and was subsequently lost. Each PQR ES cell line thus isolated and propagated originated from a single independent PQR ES cell colony.
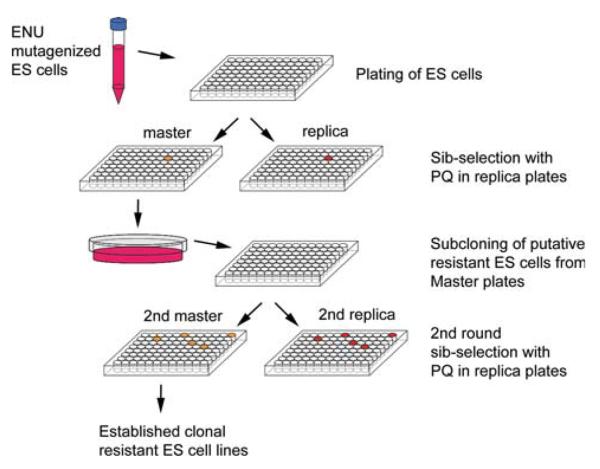
Fig. 1.
ES cell mutagenesis and selection for resistance to oxidative stress. ES cells mutagenized by ENU were plated into ten 96-well plates and a set of replica plates were prepared for “sib-selection,” while the original “masters” were frozen. Cells on the replica were treated with PQ (40 μM) to identify wells with resistant cells. Cells in the corresponding well on the master plates were then thawed and plated at low density onto culture dishes. Individual ES cell colonies were then hand-picked to a 96-well plate for a second round of sib-selection (one 96-well plate was sufficient). The resistant ES cells thus identified from the second master plate were clonally derived and free of exposure to stress or selection agents
Characterization of oxidant-resistant ES cells
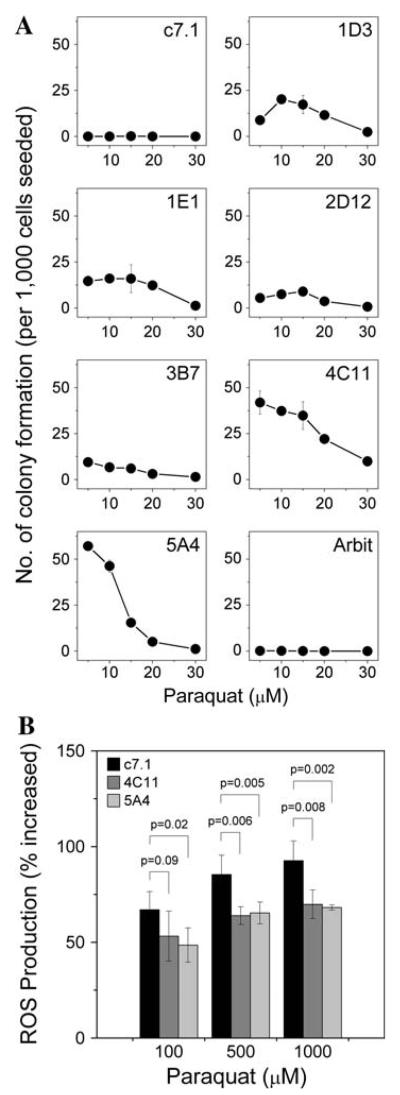
PQR ES cell clones were then subjected to treatment with PQ to confirm their resistance; all six clones were resistant to PQ (Fig. 2a) to various extents compared with parental ES cells, which are very sensitive to oxidative stress. The oxidative-resistance phenotype prompted us to measure whether ROS generation within these resistant ES cells had been altered. Two clones (4C11 and 5A4) were challenged with stressor (PQ) followed by endogenous ROS measurement with the membrane-permeable dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). Compared with the parental ES cells, both resistant ES cell clones showed less ROS in response to stressor treatment (Fig. 2b), suggesting that ROS levels in those cells was reduced, which might explain the increased resistance to oxidants.
Fig. 2.
Characterization of oxidant-resistant ES cells. a Number of resistant ES cell colonies under various PQ doses. Resistant clones (1D3, 1E1, 2D12, 3B7, 4C11, 5A4) exhibit PQ resistance to various extents compared with either the parental ES cells (c7.1) or an arbitrarily selected ENU-mutagenized ES cell clone (Arbit). Each data point represents a mean ± SD (n = 3). b Total reactive oxygen species (ROS) production in ES cells during oxidative-stress treatment. Upon PQ treatment, parental ES cells (c7.1) showed increased ROS in a dose-dependent manner. Resistant ES cell clones 4C11 and 5A4, on the other hand, showed a less pronounced increase in ROS upon PQ treatment. The percentage increase in ROS in 4C11 and 5A4 ES cells treated with 500 μM or 1 mM PQ was significantly lower than that of the parental ES cells. Error bars represent SD of means (n = 5). Significance of differences in ROS generation between parental and stress-resistant ES cells was analyzed by one-way ANOVA
Mouse production
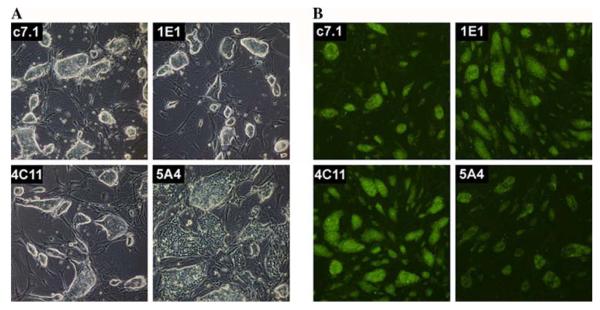
We injected ES cells from three oxidant-resistant clones (1E1, 4C11, and 5A4) into C57BL/6 J blastocysts to generate mouse chimera. Clones 1E1 and 4C11 yielded two and five chimera, respectively, but we failed to get chimera from clone 5A4 in three independent injections. The failure of clone 5A4 to produce chimeric mice may be due to its more differentiated state, as indicated by the morphology of the ES cell colonies and the reduced Oct4 level (Fig. 3). Two 4C11 and two 1E1 chimera transmitted the ES cell genome through the germline (Table 3). Thus, oxidant-resistant ES cells are able to produce germline chimera. Preserving ES cells from direct exposure to a stressor appears to be critical in that both a previous report (Martin 2005) and our initial attempts failed to yield germline transmission, while using cells from a replicate colony, unexposed to any additional stress, was quite successful. The sib-selection screening strategy described here may be employed whenever the selecting agents might affect ES cell pluripotency.
Fig. 3.
Characterization of oxidant-resistant ES cells. a Morphology of ES cell colonies in culture. Parental ES cell line c7.1 and two resistant lines (1E1 and 4C11) are germline competent and also exhibit a compacted colony morphology with a tight boundary, a characteristic typical of pluripotent ES cells. Clone 5A4, however, does not retain this morphology and, indeed, this line failed to generate mice. b Fixed ES cell colonies stained with OCT4 antibody. Compared with germline-competent clones (c7.1, 1E1, and 4C11), 5A4 appears to have lower levels of the OCT4 protein. This reduced OCT4 level may correlate with the inability of the 5A4 clone to produce chimeric mice
Table 3.
Production of chimeric mice from oxidant-resistant ES cells
| ES clone |
Injection runs |
No. of chimera obtained |
No. of germline- competent chimera |
|---|---|---|---|
| 1E1 | 1 | 2 | 2 |
| 4C11 | 1 | 5 | 2 |
| 5A4 | 3 | 0 | 0 |
Germline transmission was confirmed by genotyping in the F1 progeny. The inheritance of 129X1/SvJ allele of microsatellite markers indicates germline transmission. A total of 20 F1 pups were analyzed from each chimera breeding; failure to transmit 129X1/SvJ genome causes classification of that chimera as germline incompetent
Resistance test of mice
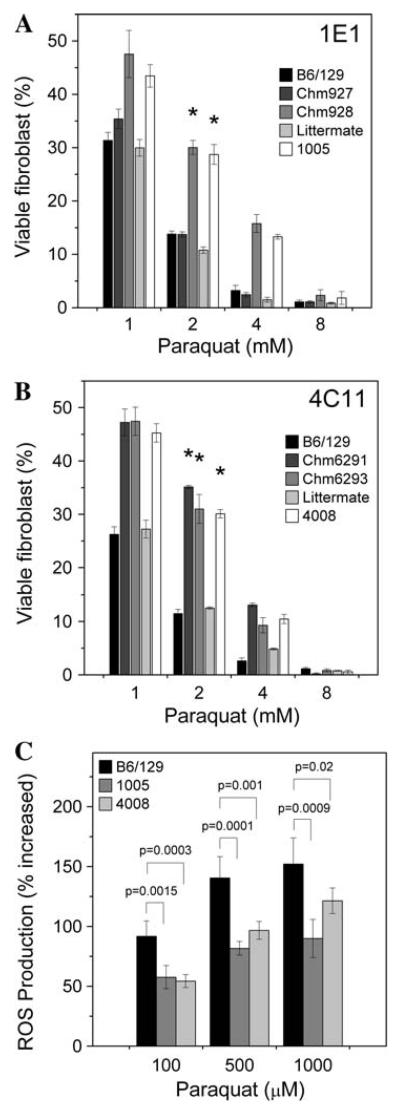
We have sought to further address the issue of whether the stress-resistant phenotype is transmitted to living mice. Skin-derived fibroblasts have been frequently cultured as a surrogate cell type to assess stress resistance of the whole animal (Harper et al. 2007; Salmon et al. 2005, 2008). This is a minimally invasive test and allows survival for subsequent tests and breeding. Indeed, stress resistance in fibroblasts correlates with longevity across several mammalian species (Kapahi et al. 1999). We isolated tail skin fibroblasts from the chimera generated from clone 1E1 to test for PQ resistance. One chimera showed enhanced PQ resistance (Fig. 4a); the other chimera had less ES cell-derived tissue, which might explain its lack of resistance. Two germline chimera generated from clone 4C11 were both PQ resistant (Fig. 4b). Two mouse lines derived from resistant ES cell clones 1E1 and 4C11 were then established by backcrossing the chimeras; the F1 progeny do not show any noticeable gross anomalies. We tested two F1 litters of the 1E1 and 4C11 chimera for PQ resistance using the fibroblast assay. We identified one and three resistant F1 mice from the 1E1 line and 4C11 line, respectively. The levels of ROS in the fibroblast induced by paraquat were also lowered compared to the normal mice (Fig. 4c), similar to what we observed in the resistant ES cells. Based on this assay we conclude that the PQR trait is recapitulated in the chimera, a mosaic animal, whose apparent resistance depends on the extent of ES cell contribution in the tissue tested (fibroblast in this case). The recovery of PQR F1 progeny indicates that the PQR phenotype is heritable, allowing lines of mutants to be established for in vivo studies. As ENU induced random point mutations throughout the genome, we do not know the genotype of the stress-resistant mice at this point. Resistant mice, however, could be identified by the described resistance test on their tail skin-derived fibroblasts for linkage analysis and subsequent gene mapping. Because we observed the PQR phenotype in the chimera and in the F1 generation, the mutations appear to be dominant. We are currently backcrossing the resistant F1 mouse to produce F2 progeny from which 50% should be resistant, as expected for a dominant mutation (assuming a monogenic phenotype). Backcrossing of the resistant mice is also necessary to segregate unrelated mutations before meaningful genetic and physiological studies would take place.
Fig. 4.
Assessment of stress resistance in skin-derived fibroblasts. Paraquat resistance of fibroblasts isolated from the tail skin of chimeric mice derived from injection of 1E1 and 4C11 ES cells. Fibroblasts (passage 3) were treated with PQ for 6 h in culture. After 20 h of recovery, cell viability was measured by the WST-1 cell proliferation assay. Untreated fibroblasts were normalized to 100% viability. a Fibroblasts from the tail of chimera (928) and an F1 male (1005) offspring were more resistant to PQ than either B6/129 F1 hybrid mice controls or five littermates (Littermate, only one is shown). b Fibroblasts from two chimera (6291 and 6293) and an F1 male mouse (4008) were resistant to PQ compared with fibroblasts from B6/129 hybrid mice and two littermates (Littermate, only one was shown). * P<0.0001 (survival measured at 2 mM PQ) between a group of six B6/129 control mice and the resistant mouse, evaluated by one-way ANOVA. Error bars represent SD of means (n = 3). c ROS production in fibroblasts during oxidant challenge. ROS levels in fibroblasts from F1 mice (1005 and 4008) treated with 100, 500, and 1000 μM of PQ were significantly less than that in fibroblasts isolated from wild-type controls (B6/129, only one is shown), as evaluated by one-way ANOVA. Error bars represent SD of means (n = 5)
Discussion
We have reported here a method for selection for a phenotype of interest using mouse ES cells. Previous studies suggested that genetically engineered ES cells might have shared a common phenotype with the mice derived, as measured by gene expression profiling (Symula et al. 2004). An earlier study, using multipotent embryonal carcinoma cell lines, had demonstrated the feasibility of selection of multipotent clones that were resistant to oxidative stress and the expression of that phenotype in differentiated progeny; the potential for the synthesis of mice with such phenotypes using ES cells was noted (Ogburn et al. 1994). Our results now provide direct evidence that a related selected phenotype (resistance to paraquat) could indeed be transmitted to the adult mice; cellular analysis of the tail tissue isolated from the animal exhibits the trait we observed in ES cells. Importantly, this procedure preserves ES cell pluripotency; selected ES cells generate germline chimera and are propagated into a living mouse carrying the phenotype selected in cell culture. For some traits, such as those discussed here, these results have profound implications; they open the possibility of direct screening for phenotypes of interest using mass selection, which has hitherto not been possible in mammals. The transmission of mutant ES cell phenotypes to adult mice would allow mouse geneticists to conduct high-throughput genetic screens to generate novel mouse mutants, parallel to the methods used in lower organisms. Although transmission of a single phenotype was demonstrated here, the success of this experiment should encourage others to further exploit this novel method for creating mouse mutants with desired phenotypes. In addition to oxidative-stress resistance, one may adapt this method by designing and implementing a suitable screening strategy to isolate ES cell clones carrying other phenotypes of interest, followed by mouse production. In a proof-of-concept setting, we used ENU to mutagenize ES cells because of its very high mutagenicity, allowing saturation of the genome with newly induced mutations. While ENU mutagenesis offers certain advantages by inducing point mutations, the use of transposon-mediated mutagenesis will significantly facilitate the identification of the mutated gene(s) conferring the phenotype by providing a molecular tag on the integration sites. Although transposon mutagenesis [e.g., PiggyBac (Wang et al. 2008)] has not achieved levels comparable to ENU, its advantage for gene identification might offset the necessity for screening a larger pool of ES cells.
Acknowledgments
We thank Ms. Irene Choi for her assistance in immunostaining experiments. This work was supported by Scientific Opportunity Funds from the Longevity Consortium (AG023122) to WSC and TEJ, an Ellison Medical Foundation Senior Scholar Award to TEJ, and RO1NS45748 to MP.
References
- Botstein D, Maurer R. Genetic approaches to the analysis of microbial development. Annu Rev Genet. 1982;16:61–83. doi: 10.1146/annurev.ge.16.120182.000425. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yee D, Dains K, Chatterjee A, Cavalcoli J, et al. Genotype-based screen for ENU-induced mutations in mouse embryonic stem cells. Nat Genet. 2000;24:314–317. doi: 10.1038/73557. [DOI] [PubMed] [Google Scholar]
- Chick WS, Mentzer SE, Carpenter DA, Rinchik EM, Johnson D, et al. X-ray-induced deletion complexes in embryonic stem cells on mouse chromosome 15. Mamm Genome. 2005;16:661–671. doi: 10.1007/s00335-005-0011-5. [DOI] [PubMed] [Google Scholar]
- de Castro E, Hegi de Castro S, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Guo G, Wang W, Bradley A. Mismatch repair genes identified using genetic screens in Blm-deficient embryonic stem cells. Nature. 2004;429:891–895. doi: 10.1038/nature02653. [DOI] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Justice MJ, Noveroske JK, Weber JS, Zheng B, Bradley A. Mouse ENU mutagenesis. Hum Mol Genet. 1999;8:1955–1963. doi: 10.1093/hmg/8.10.1955. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–771. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- Martin GM. Genetic engineering of mice to test the oxidative damage theory of aging. Ann NY Acad Sci. 2005;1055:26–34. doi: 10.1196/annals.1323.005. [DOI] [PubMed] [Google Scholar]
- Munroe RJ, Bergstrom RA, Zheng QY, Libby B, Smith R, et al. Mouse mutants from chemically mutagenized embryonic stem cells. Nat Genet. 2000;24:318–321. doi: 10.1038/73563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Ogburn CE, Turker MS, Kavanagh TJ, Disteche CM, Smith AC, et al. Oxygen-resistant multipotent embryonic carcinoma cell lines exhibit antimutator phenotypes. Somat Cell Mol Genet. 1994;20:361–370. doi: 10.1007/BF02257453. [DOI] [PubMed] [Google Scholar]
- Perla V, Perrin NA, Greenlee AR. Paraquat toxicity in a mouse embryonic stem cell model. Toxicol In Vitro. 2008;22:515–524. doi: 10.1016/j.tiv.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, et al. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Sadighi Akha AA, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Gerontol A Biol Sci Med Sci. 2008;63:232–241. doi: 10.1093/gerona/63.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symula DJ, Zhu Y, Schimenti JC, Rubin EM. Functional annotation of mouse mutations in embryonic stem cells by use of expression profiling. Mamm Genome. 2004;15:1–13. doi: 10.1007/s00335-002-2228-x. [DOI] [PubMed] [Google Scholar]
- Wang W, Lin C, Lu D, Ning Z, Cox T, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K, Horie K, Kondoh G, Kouno M, Maeda Y, et al. Genome-wide phenotype analysis in ES cells by regulated disruption of Bloom's syndrome gene. Nature. 2004;429:896–899. doi: 10.1038/nature02646. [DOI] [PubMed] [Google Scholar]