Abstract
Background. Myocardial blush grade (MBG) and myocardial contrast echocardiography (MCE) are both indices for myocardial perfusion in patients with ST-elevation acute myocardial infarction (STEMI). We aimed to compare MBG with MCE in the infarct-related artery segment for assessing infarct size in patients with STEMI treated with primary percutaneous coronary intervention (PCI).
Methods. 43 patients underwent successful (postprocedural TIMI flow 3) primary PCI for STEMI. MBG was assessed at the end of the PCI procedure and MCE was assessed 1.7±1.8 days after PCI. Enzymatic infarct size was estimated by measurementof enzyme activities by using lactate dehydrogenase (LDH) as the referenceenzyme. Cumulative enzyme release (LDHQ48) from at least five serial measurements up to 48 hours after symptom onset was calculated. Also peak creatine kinase, CK-MB and peak LDH were measured.
Results. MBG 0/1, 2 and 3 were observed in 14, 12 and 17 patients, respectively, and was compared with tertiles of MCE. We found a parallel correlation between both MBG and MCE and LDHQ48. However, there was no correlation between MCE and MBG. Patients with both normal MCE and a normal MBG had least myocardial damage and those with both impaired MCE and an impaired MBG had most myocardial damage.
Conclusion. Both MBG and MCE are good predictors of infarct size in STEMI patients treated with PCI. However, these markers are not mutually related, possibly due to time-related changes in myocardial perfusion. Combining these two markers may yield a more accurate prediction of final myocardial damage. (Neth Heart J 2010;18:25-30.)
Keywords: ST-segment elevation myocardial infarction, myocardial blush grade, myocardial contrast echocardiography, infarct size
Epicardial coronary artery patency and thrombolysis in myocardial infarction (TIMI) flow cannot be used as reliable markers of myocardial tissue perfusion after reperfusion therapy. Despite a normal coronary patency, tissue perfusion may be impaired or absent.1,2 Myocardial blush grade (MBG) has been well validated as an angiographic technique to assess myocardial perfusion in patients with ST-elevation acute myocardial infarction (STEMI).3,4 It is strongly related to prognosis in patients undergoing primary coronary intervention (PCI) for STEMI.5,6
Experimental studies showed that myocardial contrast echocardiography (MCE) correlates with myocardial perfusion.7 Several studies have shown that MCE can provide important diagnostic and prognostic information in patients with acute myocardial infarction, and predicts infarct size, myocardial viability, collateral circulation and success of reperfusion.8-11 A previous study reported that MCE, compared with MBG, peak creatine kinase (CK) and ST-segment resolution, is the best marker and most accurate measure of reperfusion at a microvascular level and an excellent predictor of LV function at one month following acute myocardial infarction.12 However, this study was hampered by a small sample size (only 15 patients were included).
In the present study, we compared MCE with MBG for assessing enzymatic infarct size in 43 patients with STEMI treated with primary PCI.
Methods
A total of 43 consecutive patients with STEMI treated with primary PCI were enrolled in this prospective study. All patients presented within 12 hours of symptom onset. They had chest pain lasting more than 30 minutes and ST-segment elevation >2 mm in the precordial leads or >1 mm in the limb leads in at least two contiguous leads.
Coronary angiography
Coronary angiography and primary coronary intervention were performed according to standard procedures and were analysed by an independent core laboratory (DIAGRAM, Zwolle, the Netherlands). Myocardial blush grade was assessed after primary angioplasty, as previously described:5
grade 0/1: no myocardial blush or minimal myocardial blush or contrast density;
grade 2: moderate myocardial blush or contrast density but less than that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery;
grade 3: normal myocardial blush or contrast density, comparable with that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery.
Echocardiography and myocardial contrast echocardiography
Echocardiography and MCE were performed as previously described.12 To assess regional and global left ventricular function (wall motion) a phased array transducer was used switched to harmonic mode, with mean transmit and receiving frequencies of 1.8 and 3.6 MHz. MCE was performed by using intravenous Optison (Molecular Biosystems, San Diego, California, USA) as the myocardial contrast agent. Commercially available equipment (Hewlett Packard Sonos 5500, Andover, Massachusetts, USA) for the echocardiographic imaging, with the patient in the left lateral decubitus position, was used. Contrast echocardiography was performed using low mechanical index real time imaging (power modulation) with a broad band 2.2 MHz phased array (range 1.8 to 2.4 MHz). Transmitted power was adjusted to produce a mechanical index of 0.1 to 0.2. Before contrast was injected, a sequence of images was captured. These included three apical views (apical two- three- and four-chamber views) and two parasternal views (long- and short-axis views) to allow baseline wall motion assessment. Optison was injected as a slow bolus (0.3 ml) through a peripheral vein, followed by a 10 ml slow saline flush (over 10 seconds). Image acquisition was initiated just before contrast injection. Manually triggered transient high mechanical index imaging (‘flash’ imaging) was used at peak contrast intensity to destroy microbubbles within the myocardium, exclude artefacts, and observe myocardial replenishment. MCE image acquisition was obtained for 10 to 15 beats following flash imaging, in the apical two-, three-, and four-chamber views. All images were stored on optical disk and on super-VHS tape for subsequent analysis. MCE was graded as follows: 1: normal; 2: reduced; and 3: absent perfusion; scores were added and divided by the number of regions analysed within the infarct-related artery segment.12
Enzyme release, enzymatic infarct size and outcome
Blood samples were obtained on admission and every 6 to 12 hours hereafter for up to 72 hours. Enzymatic infarct size (LDHQ48) was calculated based upon enzyme concentrations of lactate dehydrogenase (LDH) as the reference enzyme, in which an area under the curve was calculated from at least five measurements. A two-compartment model was used, which has been validated in studies on the turnover of radiolabelled plasma proteins and circulating enzymes.13-15 In addition, as an alternative assessment of enzymatic infarct size, peak CK was defined as the highest level of CK during admission. We also analysed major adverse cardiac event (MACE), which is defined as death, re-infarction or re-PCI at one year.
Statistical analysis
Statistical analysis was done using SPSS Science software, Chicago, Illinois, USA, version 12.0. Continuous variables were compared using ANOVA. Results are expressed as mean (SD) or as percentages. A probability value of p<0.05 was considered significant.
Results
Baseline characteristics
Patient characteristics are outlined in table 1. Mean age was 60 years; 74% were males. Five patients (12%) had a history of previous myocardial infarction.
Table 1.
Baseline characteristics.
| Variable | |
|---|---|
| Age, mean ± SD | 60±11 |
| Male, n (%) | 32 (74) |
| History of | |
| - Myocardial infarction, n (%) | 5 (12) |
| - Hypertension, n (%) | 11 (26) |
| - Diabetes, n (%) | 6 (14) |
| - Hypercholesterolemia, n (%) | 7 (16) |
| - Family history of CAD, n (%) | 16 (37) |
| - Smoking (%) | 21 (49) |
| Angiographic parameters | |
| Number of affected vessels | |
| - One-vessel disease, n (%) | 22 (51) |
| - Two-vessel disease, n (%) | 13 (30) |
| - Three-vessel disease, n (%) | 8 (19) |
| Infarct-related vessel | |
| - RCA, n (%) | 14 (32.6) |
| - LAD, n (%) | 24 (55.8) |
| - CX, n (%) | 5 (11.6) |
| Myocardial blush grade | |
| - 0/1, n (%) | 14 (32.6) |
| - 2, n (%) | 12 (27.9) |
| - 3, n (%) | 17 (39.5) |
CAD=coronary artery disease, RCA=right coronary artery, LAD=left anterior descending artery, CX=circumflex artery.
Angiography
The angiographic parameters are shown in table 1. The left anterior descending, right coronary, and circumflex coronary arteries were the infarct-related vessels in 24, 14 and 5 patients, respectively. Two or more vessels were diseased in 21 patients (49%). Angioplasty and stenting were carried out in all the patients. All patients had a TIMI grade 3 flow after the procedure. MBG was 0/1, 2 and 3 in 14, 12 and 17 patients, respectively (table 1).
Echocardiography
Echocardiography was carried out at a mean of 1.7±1.8 days after admission. Mean MCE was 1.65±0.56, median=1.7; 1st tertile: <1.50; 2nd tertile: 1.50 to 1.81, and 3rd tertile: >1.81.
Enzymatic infarct size
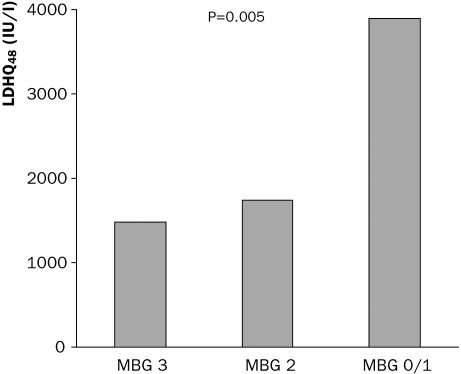
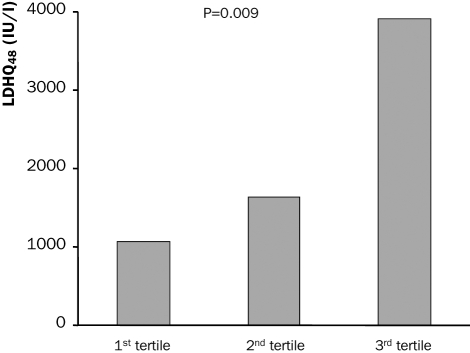
Enzymatic infarct size is shown in tables 2 and 3. LDHQ48 was significantly higher in patients with impaired MBG, 3895±2649, 1370±1299 and 1475±1086; p=0.005, in patients with MBG 0/1, 2 and 3 respectively (figure 1). Also peak CK and peak LDH were higher in patients with impaired MBG. Patients with MBG 3 had a better left ventricular ejection fraction as compared with those with a lower MBG (table 2). Enzymatic infarct size was significantly higher in patients with 2nd and 3rd tertiles of MCE as compared with the 1st tertile (table 3, figure 2). Also peak and mean CK and peak and mean LDH were significantly associated with MCE (table 3).
Table 2.
Relation between myocardial blush grade and infarct size.
| Variable | Blush 0/1 | Blush 2 | Blush 3 | P |
|---|---|---|---|---|
| LDHQ48 | 3895±2649 | 1370±1002 | 1299±1086 | 0.005 |
| CK max | 4955±3547 | 2363±1724 | 2417±2110 | 0.016 |
| CK mean | 2167±1325 | 1153±913 | 1196±952 | 0.027 |
| CK-MB max | 461±341 | 292±204 | 263±222 | 0.10 |
| CK-MB mean | 184±110 | 137±96 | 125±103 | 0.29 |
| LDH max | 2420±1462 | 1355±715 | 1217±728 | 0.005 |
| LDH mean | 1693±986 | 942±467 | 887±460 | 0.004 |
| EF | 47±11 | 47±6 | 55±10 | 0.026 |
LDH=lactate dehydrogenase, CK=creatine kinase, CK-MB=creatine kinase myocardial band, EF=ejection fraction.
Table 3.
Relation between myocardial contrast echocardiography (MCE) and infarct size.
| Variable | MCE 3rd tertile | MCE 2nd tertile | MCE 1st tertile | P |
|---|---|---|---|---|
| LDHQ48 | 3914±2798 | 1638±1260 | 1060±778 | 0.009 |
| CK max | 5172±3682 | 2267±1586 | 2062±1093 | 0.002 |
| CK mean | 2210±1487 | 1081±742 | 1181±656 | 0.009 |
| CK-MB max | 536±344 | 240±146 | 211±127 | 0.001 |
| CK-MB mean | 215±131 | 109±60 | 117±76 | 0.006 |
| LDH max | 2431±1472 | 1339±692 | 1054±413 | 0.002 |
| LDH mean | 1688±1006 | 953±426 | 780±257 | 0.002 |
| EF | 51±10 | 49±10 | 51±11 | 0.90 |
LDH=lactate dehydrogenase, CK=creatine kinase, CK-MB=creatine kinase myocardial band, EF=ejection fraction.
Figure 1.
Relation between myocardial blush grade (MBG) and enzymatic infarct size.
Figure 2.
Relation between myocardial contrast echocardiography (MCE) percentiles and enzymatic infarct size.
Relation between MBG and MCE
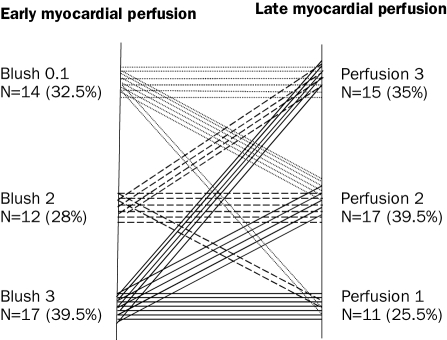
Figure 3 shows the relation between MBG and MCE. From the 17 patients with MBG 3, only seven had MCE in the 1st tertile and five patients had MCE in the 3rd tertile. From the 14 patients with MBG 0/1 six patients had MCE in the 3rd tertile and two patients had MCE in the 1st tertile. From the 15 patients with MCE in the 1st tertile, seven patients had MBG 3 and two patients had MBG 0/1. From the 15 patients with MCE in the 3rd tertile, six patients had MBG 0/1 and five patients MBG 3. There was no relation between MBG and MCE (Pearson correlation r=0.17, p=0.8). Patients with MBG 3 and MCE in the 1st tertile had a lower enzymatic infarct size and a higher LVEF as compared patients with more impaired MBG and MCE. However, patients with MBG 3 but MCE in the 2nd or 3rd tertile had a larger infarct size compared with those with MBG <3 and MCE in the 1st tertile (table 4).
Table 4.
Relation between combined MBG plus MCE and outcome.
| Variable | MBG 3/MCE≤1.5 n=9 | MBG<3MCE>1.5 n=19 | P | MBG 3/MCE>1.50 n=8 | MBG<3MCE≤1.5 n=7 | P |
|---|---|---|---|---|---|---|
| LDHQ48 | 1007±858 | 3427±2513 | 0.055 | 1543±1240 | 1008±745 | 0.47 |
| CK max | 1726±1060 | 4374±3376 | 0.031 | 3194±2754 | 2088±1140 | 0.34 |
| CK mean | 911±511 | 1848±1365 | 0.058 | 1516±1246 | 1295±770 | 0.69 |
| CK-MB max | 176±109 | 442±318 | 0.023 | 361±279 | 222±122 | 0.25 |
| CK-MB mean | 87±41 | 173±110 | 0.034 | 169±135 | 132±86 | 0.56 |
| LDH max | 927±389 | 2238±1353 | 0.009 | 1544±899 | 1087±415 | 0.24 |
| LDH mean | 706±269 | 1551±925 | 0.013 | 1091±558 | 789±230 | 0.20 |
| Ejection fraction (%) | 53±10 | 39±15 | 0.024 | 53±20 | 51±8 | 0.88 |
| MACE (%) | 11.1 | 26.3 | 0.36 | 25 | 28.6 | 0.88 |
LDH=lactate dehydrogenase, CK=creatine kinase, CK-MB=creatine kinase myocardial band, EF=ejection fraction, MACE=major adverse cardiac event (death, re-infarction or re-PCI).
Figure 3.
Relation between myocardial contrast echocardiography and myocardial blush grade. Each line represents one patient.
There was a trend towards a lower rate of MACE in patients with a normal MBG and/or MCE compared with those with an impaired MBG and/or MCE (tables 2 to 4).
Discussion
This study shows that myocardial blush grade and myocardial contrast echocardiography, two markers of myocardial perfusion, are both good predictors of myocardial infarct size in patients with STEMI treated with primary PCI. However, a correlation between MBG and MCE was not found in our study.
MBG, MCE and myocardial damage
We previously reported that MBG is related to TIMI flow in the epicardial vessel. However, almost one third of patients with TIMI 3 flow have MBG 0/1, indicating poor perfusion at the tissue level. This impaired myocardial perfusionis associated with relatively more extensivenecrosis and, as a consequence, is a predictor of poor regional and global contractile function, with predictive power beyond TIMI flow.5,8,16 Myocardial contrast echocardiography is another means of assessing myocardial perfusion and predicts infarct size, myocardial viability, collateral circulation and success of reperfusion.7-11 It has been suggested that MCE is an even better marker of myocardial perfusion.12
In our study, both MBG and MCE were good predictors of myocardial damage after STEMI. Although patients with a normal MBG more often had a normal MCE, there was no significant correlation between MCE and MBG. This may be due to several reasons. First, the scoring systems of MBG and MCE are inherently different; MBG is scored in one single projection of the heart while MCE is assessed using multiple views. Because of this, MBG might be mistakenly graded as normal due to perfusion from a neighbouring blood vessel, e.g. the right descending posterior branch and the right posterolateral artery. Moreover, MBG is a score using only four discrete values as overall outcome for the whole infarct territory, while MCE is a three-point score, averaging overall infarcted segments. This allows a more precise description of perfusion using MCE; however, in our study this did not result in a better prediction of myocardial damage. Second, MBG was determined at the end of the PCI procedure while echocardiography was performed 1.7±1.8 days later. It has been reported that after reperfusion therapy, myocardial perfusion is dynamic in nature and microvascular damage may be reversible, even in an area of initial no-reflow.17-19 The window for salvage of the myocardium may therefore be greater for some patients following acute myocardial infarction. Conversely, initially optimal reperfusion of the myocardium may worsen during follow-up.20,21 Another possible explanation of the difference in MBG and MCE may be the treatment following PCI. Treatment with antithrombotics may positively affect late myocardial perfusion (MCE) in some patients with impaired early myocardial perfusion (MBG). Conversely, delayed distal embolisation, inflammation and oedema may worsen initially adequate perfusion.
Unexpectedly, we found comparable conserved LV function in patients with normal MCE compared with those with impaired MCE (table 3). However, the end-systolic, end-diastolic volume and wall motion score were higher in patients with impaired MCE compared with those with normal MCE (data not shown).
Combining MBG and MCE
Prediction of myocardial damage after STEMI may not be accurate when only MBG or MCE is taken into account. Combining both parameters may be more predictive. As shown in table 4, patients with both adequate MCE (1st tertile) and normal MBG (grade 3) had the smallest enzymatic infarct size, and those with both impaired MBG (grade 0/1 or 2) and MCE (2nd or 3rd tertile) had the largest enzymatic infarct size. No significant difference in enzymatic infarct size was found between patients with an adequate MCE but impaired MBG versus those with an impaired MCE but normal MBG. However, patients with impaired MBG but adequate MCE had an enzymatic infarct size and rest LV function similar to those with both adequate MCE and MBG. In our study there was a trend towards a lower rate of MACE in patients with a normal MBG and/or MCE compared with those with an impaired MBG and/or MCE.
Limitation
MBG and MCE were not simultaneously assessed; MCE was obtained 1.7±1.8 days after MBG assessment. Furthermore, only one single measurement of MBG and MCE was performed. There was a trend towards a lower MACE in patients with a normal MBG and/or MCE compared with those with an impaired MBG and/or MCE (tables 2 to 4); however, a larger sample size study is needed to detect significant difference in MACE between the different groups. Finally, LAD was most common as infarct-related vessel; this may be due to selection.
Conclusion
MBG assessed acutely and MCE obtained after one to two days are both good predictors of enzymatic infarct size in STEMI patients treated with PCI. However, these markers are not mutually related. Combining these two markers may yield a more accurate prediction of final myocardial damage.
References
- 1.Bowers TR, O'Neill WW. Beyond TIMI III flow. Circulation. 2000;101:2332–4. [DOI] [PubMed] [Google Scholar]
- 2.Kaul S. Coronary angiography cannot be used to assess myocardial perfusion in patients undergoing reperfusion for acute myocardial infarction. Heart. 2000;86:483–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van 't Hof AWJ, Hoorntje JCA, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002:23:1112–7. [DOI] [PubMed] [Google Scholar]
- 4.Haager PK, Christott P, Heussen N, Lepper W, Hanrath P, Hoffmann R. Prediction of clinical outcome after mechanical revascularization in acute myocardial infarction by markers of myocardial reperfusion. J Am Coll Cardiol. 2003:41:532–8. [DOI] [PubMed] [Google Scholar]
- 5.Van 't Hof AWJ, Liem A, Suryapranata H, Hoorntje JCA, de Boer MJ, Zijlstra F, on behalf of the Zwolle Myocardial Infarction Study Group. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction. Circulation. 1998;97:2302–6. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol. 2002:39:591–7. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva FS, Glasheen WP, Sklenar J, Kaul S. Assessment of risk area during coronary occlusion and infarct size after reperfusion with myocardial contrast echocardiography using left atrial and right atrial injections of contrast. Circulation. 1993;86:596–604. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the “no reflow” phenomenon. Circulation. 1996;93:223–8. [DOI] [PubMed] [Google Scholar]
- 9.Sabia PJ, Powers ER, Jayaweera AR, Ragosta M, Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction: A study using myocardial contrast echocardiography. Circulation. 1992;85:2080–9. [DOI] [PubMed] [Google Scholar]
- 10.Sabia P, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31. [DOI] [PubMed] [Google Scholar]
- 11.Ragosta M, Camarano G, Kaul S, Powers ER, Sarembock IJ, Gimple LW. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation. 1994;89:2562–9. [DOI] [PubMed] [Google Scholar]
- 12.Greaves K, Dixon SR, Fejka M, O'Neill WW, Redwood SR, Marber MS, et al. Myocardial contrast echocardiography is superior to other known modalities for assessing myocardial reperfusion after acute myocardial infarction. Heart. 2003;89:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Laarse A, Hermens WT, Hollaar L, Jol M, Willems GM, Lemmers HE, et al. Assessment of myocardial damage in patients with acute myocardial infarction by serial measurement of serum alpha-hydroxybutyrate dehydrogenase levels. Am Heart J. 1984;107:248–60. [DOI] [PubMed] [Google Scholar]
- 14.Hermens WT, Willems GM, Nijssen KM, Simoons ML. Effect of thrombolytic treatment delay on myocardial infarct size [letter]. Lancet. 1992;340:1297. [DOI] [PubMed] [Google Scholar]
- 15.de Boer MJ, Suryapranata H, Hoorntje JCA, Reiffers S, Liem AL, Miedema K, et al. Limitation of infarct size and preservation of left ventricular function after primary coronary angioplasty compared with intravenous streptokinase in acute myocardial infarction. Circulation. 1994;90:753–61. [DOI] [PubMed] [Google Scholar]
- 16.Iliceto S, Marangelli V, Marchese A, Amico A, Galiuto L, Rizzon P. Myocardial contrast echocardiography in acute myocardial infarction: pathophysiological background and clinical applications. Eur Heart J. 1996;17:344–53. [DOI] [PubMed] [Google Scholar]
- 17.Kamp O, Lepper W, Vanoverschelde JL, Aeschbacher BC, Rovai D, Assayag P, et al. Serial evaluation of perfusion defects in patients with a first acute myocardial infarction referred for primary PTCA using intravenous myocardial contrast echocardiography. Eur Heart J. 2001;22:1485–95. [DOI] [PubMed] [Google Scholar]
- 18.Korosoglou G, Labadze N, Hansen A, Selter C, Giannitsis E, Katus H, et al. Usefulness of real-time myocardial perfusion imaging in the evaluation of patients with first time chest pain. Am J Cardiol. 2004;94:1225–31. [DOI] [PubMed] [Google Scholar]
- 19.Badano LP, Werren M, Di Chiara A, Fioretti PM. Contrast echocardiographic evaluation of early changes in myocardial perfusion after recanalization therapy in anterior wall acute myocardial infarction and their relation with early contractile recovery. Am J Cardiol. 2003;91:532–7. [DOI] [PubMed] [Google Scholar]
- 20.Galiuto L, DeMaria AN, May-Newman K, Del Balzo U, Ohmori K, Bhargava V, et al. Evaluation of dynamic changes in microvascular flow during ischemia-reperfusion by myocardial contrast echocardiography. J Am Coll Cardiol. 1998;32:1096–101. [DOI] [PubMed] [Google Scholar]
- 21.Pierard LA. Assessing perfusion and function in acute myocardial infarction: how and when? Heart. 2003;89:701–3. [DOI] [PMC free article] [PubMed] [Google Scholar]