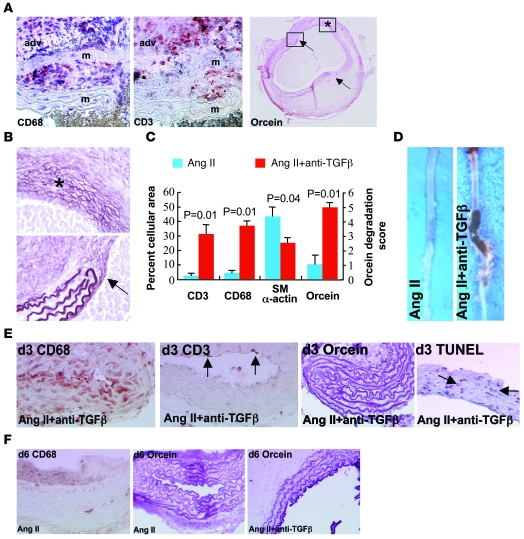
Figure 3. Characterization of Ang II–induced aortic aneurysms in mice treated with anti–TGF-β antibody.
(A) Representative photomicrographs of macrophage (CD68), T lymphocyte (CD3), and elastin (orcein) stainings of aortic aneurysms from mice infused with Ang II and treated with anti–TGF-β antibody. adv, adventitia; m, media. Arrows show the sites of complete elastin degradation. (B) Higher magnifications of vessel areas indicated in the right panel of A. Asterisk indicates advanced elastin disruption of all lamellae. Arrow shows the edge of degraded elastin lamellae. (C) Quantification (n = 5 mice per group) of smooth muscle cell (α-actin) content, elastin (orcein) degradation, as well as macrophage and T lymphocyte infiltration. (D) Representative photomicrographs of aortas recovered from mice infused with Ang II in the presence or absence of anti–TGF-β antibody. (E) Aortic tissue sections recovered after 3 days of treatment and stained for the presence of macrophages (CD68, brown), T lymphocytes (CD3, punctuate red-brown, arrows), elastin (orcein), or apoptotic cells (TUNEL, red-brown nuclei, arrows). Note that macrophage infiltration preceded T cell accumulation and overt elastin degradation and coincided with the occurrence of VSMC death. (F) Staining for macrophages (CD68) and elastin (orcein) after 6 days of treatment. Rare macrophages and no elastin degradation were observed in the Ang II group. However, intense elastin degradation (2.4 ± 0.4 elastin lamellae lost) was detected at this time point in the Ang II + anti–TGF-β group. Data are representative of 5 mice per time point and per group. Original magnification, ×400 (A, except right panel: ×40); ×400 (B); ×100 (E, except right panel: ×200); ×200 (F).