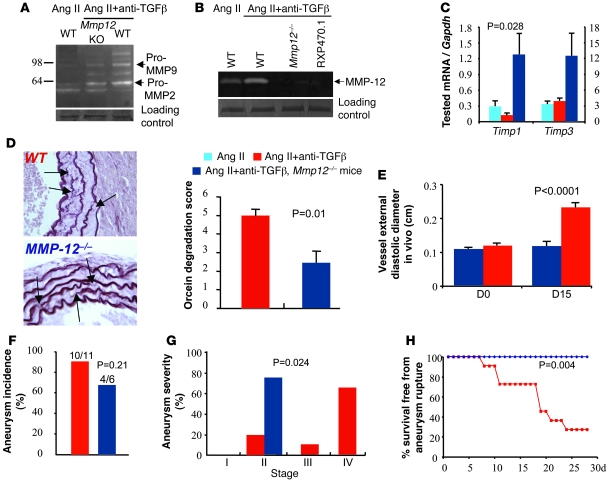
Figure 7. MMP-12 is required for aneurysm progression and rupture in mice treated with Ang II and anti–TGF-β antibody.
Representative examples of 5 separate experiments showing gelatinolytic MMP-9 and MMP-2 (A) and caseinolytic MMP-12 (B) activities in suprarenal abdominal aortas of mice infused with Ang II, with or without anti-TGF-β, and with or without genetic deficiency for Mmp12. RXP470.1 completely inhibited the caseinolytic activity, further indicating that the activity was specific for MMP-12. Loading controls represent bands obtained after Coomassie blue staining. For technical reasons, loading control of the casein zymography was run on a separate gel. (C) Quantification of aortic mRNA expression (Q-PCR) of Timp1 and Timp3 (n = 5–6 per group). We found a significant increase in Timp1 expression in Mmp12-deficient mice. (D) Representative examples and quantitative analysis of elastin (orcein staining) degradation (arrows) in WT and Mmp12-deficient mice infused with Ang II and treated with anti–TGF-β antibody. Data were collected using aortic specimens recovered at day 28 or at necropsy when animal death from AAA rupture occurred before day 28. Original magnification, ×400. (E) Vessel diameter in vivo using ultrasound imaging. Targeted M-mode–derived blinded measurements of aortic diameters were obtained as described in Methods at days 0 and 15 after the beginning of treatment. Vessel dilatation at the suprarenal level was abrogated at day 15 in Mmp12-deficient mice. (F–H) Quantification of aortic aneurysm incidence (F) and severity (G) as well as Kaplan-Meier curves of survival free from aneurysm rupture (H) in WT and Mmp12–deficient mice. Mmp12 deficiency significantly reduced aneurysm severity and completely protected from aneurysm rupture.